
Laboratory Accreditation Manual
Richard C. Friedberg, MD, PhD, FCAP
Editor
PATIENT SAFETY
COMPLIANCE
CONSISTENCY
CONFIDENCE
ACCURACY
QUALITY
29957_LAM_CVR_Bk2_.indd 129957_LAM_CVR_Bk2_.indd 1 2/22/22 10:52 AM2/22/22 10:52 AM

Contacting the CAP Accreditation Programs
Laboratories may contact the LAP with questions on accreditation-related topics by:
Email: accred@cap.org.
Phone: 800-323-4040 or 847-832-7000. CAP Accreditation business hours are 8:00
AM–5:00 PM
Central Time, Monday through Friday, excluding holidays.
Mail:
CAP Accreditation Programs
College of American Pathologists
325 Waukegan Road
Northfield, IL 60093
For specific accreditation topics, laboratories can submit questions to accred@cap.org or contact the CAP
accreditation staff by phone (800-323-4040 or 847-832-7000) at the extensions listed below.
Topic
Extension
• Interpretation of checklist items
• Responding to deficiencies
• Technical review of deficiencies
6065
• Inspector demographic changes
• Reimbursement for inspection
• Specialty inspector
7380
Systems inspections
7290
Staff inspector information
7279
• Proficiency testing enrollment
• Proficiency testing participation
Performance compliance notices
• CAP-accepted proficiency testing programs
6052
Accreditation Resources in e-Lab Solutions Suite
The CAP is constantly seeking new ways to help guide your laboratory through the accreditation process. We
recently revised and expanded our resources to make it easier to find the answers you seek. New content
includes checklist Q & A’s written by technical specialists, and an informative Inspection Prep course. What’s
more, everything is fully searchable, so you can quickly find what you need. Simply log in to the e-LAB
Solutions Suite whenever you have an accreditation question.
Continued on inside back cover
College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
1
TABLE OF CONTENTS
TOPIC PAGE
Introduction 2
Accreditation Program Types 7
Accreditation Program Philosophies 12
Applying to CAP Accreditation Programs 16
Inspection Options 21
Preparing for the Inspection: Laboratory 27
Preparing for the Inspection: Inspection Team 31
Proficiency Testing: Enrollment and Handling 42
Proficiency Testing: Failures and Monitoring 46
Accreditation Checklists 51
Conducting the Inspection: General Principles and Meetings 56
After the Inspection: Inspection Team 73
After the Inspection: Laboratory 75
Maintaining Accreditation 82
Non-Routine Inspections 88
Complaints and Investigations 89
Accreditation Program Requirements for International
91
Appendices 89
Appendix A: Accreditation Checklists Overview
96
Appendix B: Instructions for Determining Test Volume
104
Appendix C: Minimum Period of Retention of Laboratory Records and
Materials (CAP Policy PP)
107
Appendix D: Glossary of Terms
115
Appendix E: CAP Accreditation Program Website Tools
130

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
2
INTRODUCTION
TOPIC PAGE
Purpose of this Manual 2
Overview of Accreditation Programs 2
Laboratory Accreditation Program (LAP) Organization 3
Commissioners 4
Inspectors and CAP Staff 4
Accreditation Documents 5
Standards for CAP Accreditation Programs 5
Accreditation Checklists 6
Purpose of this Manual
The Laboratory Accreditation Manual provides laboratories and inspectors a basic overview of
the CAP’s accreditation programs and accreditation processes.
Overview of Accreditation Programs
The College of American Pathologists accreditation programs provide an engaging, dynamic,
and collaborative process that fosters an environment of continuous improvement. A
description of each of these programs is included in the Accreditation Program Types
section.
The accreditation programs were created with the primary objective of improving the quality of
clinical laboratory services through voluntary participation, professional peer review, year-round
education, and compliance with established performance standards. Since their creation, these
programs have become widely acknowledged for excellence. In total, the CAP accredits
approximately 8,000 laboratories in 58 countries.
The accreditation programs are based on rigorous accreditation standards translated into
detailed checklist requirements that provide a clear roadmap not only to achieve accreditation
but also for running a high-quality laboratory. CAP inspection teams use the checklists as a
guide to assess the laboratory's overall management and operation. Inspectors examine
preanalytic, analytic, and postanalytic aspects of quality management (QM) in the laboratory.
These include the performance and monitoring of general quality control (QC); test
methodologies and specifications; reagents, controls, and media; equipment; specimen
handling, test reporting and internal performance assessment; and external proficiency testing.
In addition, personnel requirements, safety, document management, and other administrative
practices are evaluated in the inspection process.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
3
The programs are internationally recognized and are the only ones that utilize teams of
practicing laboratory professionals as inspectors for all laboratory disciplines. Designed to go
well beyond regulatory compliance, the programs help laboratories utilize best practices while
achieving the highest standards of excellence to have a positive impact on patient care.
Laboratory Accreditation Program (LAP) Organization
In accordance with the CAP vision and the CAP Board of Governors, the Council on
Accreditation (CoA) sets the strategic direction for the Laboratory Accreditation Programs
(LAP). The CoA establishes LAP policy, and monitors overall program effectiveness in ensuring
that participating laboratories meet regulatory and CAP requirements. The CoA is responsible
for:
• Advancement of the CAP’s accreditation programs as the prime exemplar for the
inspection and accreditation of clinical laboratories and biorepositories
• Administration of the programs through the principles of peer review and education
• Furthering the goal of laboratory improvement to ensure that quality laboratory services
are provided to patients and clients
• Continuation of the program’s ability to meet the scientific, service, and regulatory needs of
participants
• Overseeing the activities of the program regional and state commissioners
• Recognition of the pathologist laboratory director’s role in clinical decision making and
consultation.
In order to fulfill these roles, the CoA oversees and coordinates the activities of nine
accreditation program to align committee priorities and activities with the overall goals and
strategies supporting the CAP’s accreditation programs. The CoA uses the expertise of more
than 30 CAP scientific resource committees to keep the programs and their requirements up to
date with the latest best practices as new advances and technology become part of the modern
laboratory workflow. All of these committees are composed of volunteer CAP member experts
assisted and coordinated by CAP staff.
The nine accreditation program committees are:
• Accreditation Committee: responsible for ensuring objectivity and consistency in CAP
accreditation decisions and accreditation status decisions, including accreditation
suspension and probation, based on recommendations from reviewing commissioners,
technical specialists, and other LAP committees.
• Accreditation Education Committee: oversees the continual development and
implementation of all education activities that support the CAP accreditation programs.
• Biorepository Accreditation Program Committee: promotes high standards for the
procurement, processing, storage, and distribution of biospecimens that align with quality
best practices in the biorepository field to support scientific research.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
4
• CAP ISO 15189: charged with ensuring objectivity and consistency in CAP ISO 15189
accreditation decision making by centralizing the decision-making criteria and processes.
• Checklists Committee: ensures that the accreditation requirements of the laboratory
accreditation programs are a practical tool to promote high standards in pathology and
laboratory medicine and are equal to or exceed regulatory requirements.
• Complaints and Investigations Committee: oversees and adjudicates complaints,
investigations, and validation inspections.
• Continuous Compliance Committee: ensures that accredited laboratories maintain
continuous compliance with CAP and other regulatory requirements through oversight
and education in the areas of proficiency testing (PT) and other mandated quality
activities.
• Inspection Process Committee: oversees pre-inspection and inspection processes to
improve the timeliness and quality of inspections.
• International Accreditation Committee: oversees the development, support, and
growth of the accreditation programs outside of the United States.
Commissioners
Regional commissioners are responsible for the accreditation activities of a specified group of
laboratories. This may include the timely assignment of inspectors, review of inspection findings,
and presentation of accreditation issues to the Accreditation Committee. Following an inspection
(in-person or virtual), the regional commissioner and CAP technical staff review the inspection
findings and the laboratory’s corrective action, and contribute to any follow-up necessary to
reach an accreditation decision.
State and deputy state commissioners assist the regional commissioners. These volunteer
pathologists are responsible for validating proposed inspector matches for the laboratories in
their geographic regions. They are assisted by CAP staff to ensure that inspections are timely
and in accordance with accreditation program policy. They are responsible for providing
feedback and mentoring to volunteer inspectors.
Inspectors and CAP Staff
CAP inspection teams are trained, practicing laboratory professionals who manage the same
workflows and undergo the same challenges faced by the inspected laboratories. Typically, the
inspection team leader is a board-certified pathologist who has received training and has
participated in several inspections as a team member. Inspection team members are
pathologists, doctoral scientists, supervisory-level medical technologists, pathology residents
and fellows, and other individuals who have been trained in CAP inspection requirements and
have expertise in the area of the laboratory that they inspect.
The CAP accreditation program staff at the CAP headquarters in Northfield, Illinois, comprises
technical and administrative personnel who carry out the policies and procedures of the CoA
and who are responsible for the management and operation of the program. They also include a
limited number of full-time inspectors who conduct inspections meeting defined criteria.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
5
Accreditation Documents
In addition to the Laboratory Accreditation Manual (LAM), three other documents are
fundamental to the inspection process:
1) the Standards for Laboratory Accreditation (the Standards)
2) the Accreditation Checklists
3) the Inspector’s Summation Report (ISR).
Through peer review, the inspector uses the checklists to determine if the laboratory meets the
criteria set out in the Standards. The inspector collects and records information on the ISR,
which serves as; the basis for the regional commissioner’s accreditation recommendation. In
addition to verifying compliance with accreditation requirements, the inspection team typically
shares ideas for laboratory improvement. In return, the inspection team members often take
new ideas or processes back to their own laboratories.
Standards for CAP Accreditation Programs
The Standards constitute the core principles of the CAP’s accreditation programs. The objective
of the Standards is to ensure that accredited laboratories meet the needs of patients,
physicians, and other health care practitioners. The CAP accredits laboratories that conform to
the Standards. Each of the accreditation programs has its own Standards for Accreditation. The
CAP Board of Governors approves these standards, which have evolved through years of
study and continuous review by the CoA. The inspector must be familiar with each standard and
its interpretation. A copy of the Standards is included with each inspection packet and must be
reviewed before the inspection of the laboratory. The inspection team leader is considered the
on-site authority for the interpretation of these standards.
Standard I identifies the qualifications, responsibilities, and role of the laboratory director. It
discusses which responsibilities may be delegated, as well as the role of a consulting
pathologist.
Standard II addresses the physical resources of the laboratory, including space and
instrumentation; furnishings; communication and data processing systems; reagents and other
supplies; ventilation; piped gases and water; public utilities; storage and waste disposal; and
protection of patients, laboratory personnel, and visitors from hazardous conditions.
Standard III encompasses quality management. This includes discussions of test system
validations, QC of preanalytic, analytic and postanalytic processes, proficiency testing (or
periodic alternative assessments of laboratory test performance), and ongoing performance
improvement.
Standard IV includes the administrative requirements of the program. Laboratories must
comply with the requirements specified in the Standards, the terms of accreditation, and the
accreditation checklists. Inspection by a CAP-provided team and an interim self-inspection are

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
6
the cornerstones of the inspection requirement. Participating laboratories also field an
inspection team when requested.
Accreditation Checklists
Each checklist is a detailed list of requirements that the inspector uses to determine if the
laboratory meets the Standards. Each requirement is uniquely numbered and centers on a
declarative statement. The checklists serve as instruments to guide the conduct of the
inspection. The checklists are revised annually and customized for each laboratory based on its
exact testing menu. All CAP checklists minimally meet the CLIA regulations and are approved
by CMS on an annual basis. Detailed information on the checklists is included in the
Accreditation Checklists section and in Appendix A:
Accreditation Checklists Overview.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
7
ACCREDITATION PROGRAM TYPES
TOPIC PAGE
Laboratory Accreditation Program 7
Reproductive Laboratory Accreditation Program 8
Forensic Drug Testing Program 9
Biorepository Accreditation Program 10
CAP 15189™ Accreditation Program 10
Utilizing a unique peer-inspection model, annual checklist updates, and year-round education,
the CAP’s accreditation programs cover the entire spectrum of laboratory disciplines.
The CAP’s accreditation programs offer:
• Blueprint for operating a high-quality facility
• Confidence in the accuracy of clinical reports
• Improved risk management
• Access to best practices
• The right to display the CAP Accreditation Mark.
Five unique programs, specifically tailored to the type of laboratory or facility, are available.
Laboratory Accreditation Program
The Laboratory Accreditation Program (LAP) was established in 1961. The current range of
laboratory disciplines includes:
• Anatomic Pathology
• Chemistry and Toxicology
• Clinical Biochemical Genetics
• Cytogenetics
• Cytopathology
• Flow Cytometry
• Hematology
• Histocompatibility
• Immunology
• Microbiology
• Molecular Pathology
• Point-of Care Testing

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
8
• Transfusion Medicine
• Urinalysis.
The LAP accredits a wide variety of laboratories in different settings, such as community
hospitals, university-based hospitals, out-patient clinics, and reference laboratories. The
program uses a two-year accreditation cycle where laboratories have an inspection every two
years by an inspection team made up of practicing professionals.
The CAP’s comprehensive program incorporates all of the required standards from CLIA, US
Food and Drug Administration (FDA) and the US Occupational Safety and Health
Administration (OSHA). Our program will exceed the standards where doing so materially adds
to patient care and safety. The CAP retains deemed status with the US Centers for Medicare &
Medicaid (CMS), The Joint Commission, the United Network for Organ Sharing (UNOS), the
National Marrow Donor Program (NMDP), the Foundation for the Accreditation of Cellular
Therapy (FACT), and many US agencies.
Eligibility Requirements:
• The CAP accredits laboratories performing testing on clinical biological specimens
using methodologies and clinical application within the expertise of the program.
Laboratories must be appropriately licensed to perform testing when required by law.
• The LAP is available to both domestic and international laboratories. Information
specific to international laboratories interested in laboratory accreditation is available in
the Accreditation Program Requirements for International Laboratories
section.
Laboratories that are part of a healthcare system with highly integrated laboratory services may
be eligible for the System Inspection option. Refer to section Applying for Accreditation
for more
information.
Reproductive Laboratory Accreditation Program
The Reproductive Laboratory Accreditation program (RLAP) was originally designed in
collaboration with the American Society of Reproductive Medicine (ASRM) in 1993. The
services covered in the RLAP include:
• Andrology
• Limited clinical laboratory testing (eg, hormone assays, hematology, urinalysis)
• Embryology
• Cryopreservation
• Reproductive tissue storage.
Laboratories in the RLAP have an inspection every two years by an inspection team made up
of practicing professionals. They are inspected with the Reproductive Laboratory, Laboratory
General, Director Assessment, and All Common Checklists. Other discipline-specific, checklists
are added if the scope of clinical testing so warrants.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
9
The RLAP accreditation may be used to demonstrate compliance with the CLIA ’88 regulations
for andrology and other tests regulated under CLIA. The CAP has been approved
as an accrediting organization by the CMS and is recognized by The Joint Commission. In
addition, the Society of Assisted Reproductive Technology (SART) recognizes RLAP
accreditation to meet membership requirements for in vitro fertilization facilities.
Eligibility Requirements:
• Laboratories that perform at least one embryology-related procedure or perform
eligible for the RLAP.
Information specific to international laboratories interested in laboratory accreditation is available in
the Accreditation Program Requirements for International Laboratories
section.
Forensic Drug Testing Program
The Forensic Drug Testing Program (FDT) was designed for the unique needs of forensic drug
testing laboratories for nonmedical purposes. This program is not intended for medical testing
or CLIA compliance.
The program ensures confidence in the accuracy of forensic drug test results through
evaluation of checklist requirements for sample integrity and security using robust chain-of-
custody, annual validation of all methods, tight controls on test systems, and secondary review
of all confirmatory test results.
Laboratories in FDT have an inspection every two years by an inspection team made up of
practicing professionals. They are inspected with the Forensic Drug Testing, Laboratory
General, Director Assessment, and All Common Checklists.
Eligibility Requirements:
• The FDT program is intended for laboratories that perform screening and confirmatory
testing on urine, oral fluid, hair, nail, meconium, umbilical cord, and whole blood.
• The program also accepts laboratories that only screen the specimen types listed
above by nonwaived methods. Confirmatory testing must be performed by a laboratory
accredited by the CAP Forensic Drug Testing Program or one certified by the
Substance Abuse and Mental Health Services Administration.
Information specific to international laboratories interested in laboratory accreditation is
available in the Accreditation Program Requirements for International Laboratories
section.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
10
Biorepository Accreditation Program
The Biorepository Accreditation Program (BAP) is the world’s first accreditation program
designed specifically for biorepositories that collect, process, store and distribute biospecimens
for research. The goal of the program is to provide requirements for standardization in
biorepository processes that will result in high-quality specimens that can be used to support
research, drug discovery, and personalized medicine.
Diagnostic patient testing must be performed within a CLIA licensed laboratory. In 2019, the
CMS approved the CAP’s biorepository checklist requirements as being consistent with CLIA
regulations. Alignment of the checklists from the CAP’s Biorepository Accreditation Program
and Laboratory Accreditation Program help ensure:
• Confidence in specimen provenance – A clinical laboratory director may decide to
accept specimens for testing from a BAP-accredited repository because of the formal
CLIA-approved requirements in specimen collection integrity.
• Confidence in pre-analytic variable tracking and control for samples used in drug trials
with associated biomarker development
• Improved alignment of accreditation preparation and inspection processes for
repositories affiliated with CAP-accredited laboratories.
Services covered include biorepository specimen collection/procurement, specimen distribution
and agreements, specimen informatics, specimen processing, and specimen storage.
Facilities in the BAP have an inspection every two years by an inspection team made up of
practicing professionals. They are inspected with the Biorepository, Laboratory General, Director
Assessment, and All Common checklists:
Eligibility Requirements:
• Applies to facilities that receive, store, process, and/or disseminate biospecimens, their
derivatives, and relevant data for research purposes
• Not applicable to tissue storage for transplant purposes.
CAP 15189™ Accreditation Program
Accreditation to the ISO 15189 standard strengthens the quality management system
throughout the laboratory and all parts of the organization that interact with the laboratory,
enabling process improvement, risk reduction, and improved operational efficiency.
CAP 15189 accreditation complements the universally recognized best-in-class standards and
laboratory practices found in the CAP Laboratory Accreditation Program. It adds the process
rigor and quality system scope of the ISO 15189 standard, allowing the CAP to probe deeper
for system-related issues and vulnerabilities in laboratory operations.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
11
The CAP 15189 Program accredits to the ISO I5189 standard for laboratories in the United
States and Canada that have already demonstrated compliance to the CAP’s core
accreditation program (LAP). Recognizing that some accredited laboratories have core and
branch laboratories located in other countries, we offer the CAP 15189 program to multi-
national organizations who have standardized their practices across their global network to
include both the core CAP accreditation program and to the ISO 15189 standard. The CAP has
recently expanded to other markets where sufficient demand exists. Please contact the CAP at
for further information.
Laboratories in the CAP 15189 program have an on-site assessment every three years by
experienced assessors that have practical knowledge of medical laboratory testing and ISO
quality management systems auditing. The CAP program uniquely offers an assessment of the
maturity of laboratory’s quality management system, in addition to online education courses on
quality management systems.
Eligibility Requirements:
• Accreditation to ISO 15189 through the CAP is available to US-based and Canadian
medical laboratories.
• Laboratories applying to CAP 15189 must first be accredited by the CAP Laboratory
Accreditation Program.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
12
ACCREDITATION PROGRAM PHILOSOPHIES
TOPIC
PAGE
Peer Review
12
Thoroughness
12
Judgment
13
Disputes
13
Harassment
13
Solicitation
14
Confidentiality
14
Confidentiality
– HIPAA Privacy Rule and HITECH Act 14
Inspector
Liability 15
Conflict
of Interest 15
Peer Review
Purpose: To improve laboratory performance through objective evaluation and constructive
feedback.
The inspector can enhance the spirit of peer review and the educational benefit of the inspection
process by adhering to the following:
• As representatives of the CAP Accreditation Program(s), inspectors must strive to be
objective and fair. There is often more than one way to comply with a requirement.
• The inspection team leader should be a peer of the laboratory director.
• Deficiencies should be presented factually, and recommendations for improvement
may be provided if possible.
• A negative, unduly critical, or punitive attitude should be avoided.
• Deficiencies cited by the inspection team may be challenged. If resolution of a
disagreement between laboratory personnel and an inspector cannot be achieved
before or during the summation conference, the laboratory may challenge the
deficiency during the post-inspection process. For more information, refer to the section
After the Inspection: Laboratory - Challenging a Deficiency.
Thoroughness
The CAP is approved by the Centers for Medicare and Medicaid Services (CMS) as an
accrediting organization and must meet all federal regulatory requirements for those
laboratories subject to US regulations. Participating laboratories expect a thorough, detailed,

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
13
and fair inspection. All applicable items in a customized checklist must be inspected. As part of
providing quality patient care, laboratories must be inspection-ready at all times. Laboratories
appreciate validation of the work they do and deserve a comprehensive inspection. A
deficiency should not be overlooked because it seems minor.
Judgment
The CAP Accreditation Program(s) relies upon the inspector’s judgment more than any other
attribute in the assessment of a laboratory. This attribute is, however, the most difficult to
standardize. There will be occasions when a conscientious inspector will have difficulty deciding
whether a laboratory is compliant with a checklist requirement. Many of these decisions involve
assessment of partial compliance with a checklist requirement. Therefore, the inspector must
describe the observations as completely as possible in the Inspector’s Summation Report (ISR).
This description should include details of the sampling performed to assess compliance with
the requirement. For example, a description may include, “In the review of xx number of records
for a specific expected result, the laboratory was found to be noncompliant with yy records.”
With this detailed information, the CAP can better assess the corrective action that the
laboratory proposes.
Disputes
To help resolve questionable citations, the inspector and/or laboratory personnel may contact
the CAP’s accreditation technical staff by telephone during the inspection (800-323-4040 ext.
6065 or 847-832-7000). Following the inspection, if a laboratory wishes to challenge a particular
citation, it must state its disagreement in the deficiency response and provide documentation to
demonstrate how it was compliant before it was inspected. The regional commissioner will
review disputed items and determine if the deficiency can be removed from the inspection
record.
Harassment
Employees of laboratories inspected by the CAP are entitled to a workplace environment that is
free from sexual or other unlawful harassment. Prohibited harassment includes any comments,
gestures, innuendos, or physical contact that create an intimidating, offensive, or hostile
environment. Also prohibited are behaviors that harass an employee based on race, gender,
disability, age, religion, national origin, or other legally protected category.
Inspectors on a CAP team, whether the team leader or a team member, must never display
conduct that can reasonably be construed as harassment. Team leaders must ensure that the
behavior of team members is consistent with this position and must intervene actively if
inappropriate conduct is observed.
Employees of laboratories should report inappropriate conduct by CAP team leaders or team
members to CAP headquarters. The CAP does not tolerate harassment. In cases of
documented harassment, the CAP will take appropriate action.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
14
Furthermore, CAP inspectors should expect that the employees of laboratories will treat them
with equivalent respect, free from unlawful harassment, and not create an adverse experience
for the inspector. Inspection team members should also report any inappropriate conduct by
the laboratory employees to the CAP.
Solicitation
Inspectors must not in any way solicit the institution, the laboratory, or its employees for any
purpose. They must never display conduct that can be reasonably construed as a solicitation.
Inspectors should not request any information from the institution or laboratory regarding fees or
other business-related matters. The inspector should not request any information regarding the
laboratory director’s contractual relationship with the institution’s administration. However, when
the laboratory director is present less than full-time, it is appropriate to ask about contractual
agreements indirectly to ensure that the needs of the institution are met.
Confidentiality
All inspection findings are confidential and should not be discussed in any context other than the
inspection itself. Moreover, inspection findings should not be disclosed to anyone not associated
with the accreditation process unless appropriate prior documented consent has been obtained.
Confidentiality – HIPAA Privacy Rule and HITECH Act
Under the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the CAP is
considered a “business associate” of any CAP-accredited laboratory that is designated a
“covered entity” under HIPAA. The CAP is required, therefore, to enter into a Business
Associate Agreement (BAA) with such a laboratory to protect the privacy and security of patient
health information. The CAP has developed a standardized model BAA for its accredited
laboratories to meet HIPAA, the privacy and security regulations promulgated thereunder, and
Subtitle D of the Health Information Technology for Economic and Clinical Health Act of 2009
(HITECH). The model BAA may be found on
cap.org
in e-LAB Solutions Suite under
Organization Profile – Additional Information.
The CAP further protects the CAP-accredited laboratory by requiring all CAP inspectors to attest
on the inspection report that they will keep all patient information confidential and use it only for
purposes of the CAP inspection. Other CAP personnel or agents who may have access to
protected health information are trained concerning their obligation to keep this information
confidential and to use such information only within the context of the inspection and
accreditation services provided to the laboratory. In addition, the CAP requires that laboratories
submit only documentation and other materials to the CAP that have been de-identified of all
protected health information (PHI), as that term is defined in 45 C.F.R. Parts 160 and 164, in
accordance with HIPAA and its implementing regulations (see 45 C.F.R. § 164.514(b)) unless
the laboratory must submit PHI to the CAP in order to respond to a deficiency or complaint
investigation.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
15
Inspector Liability
The CAP bylaws include a provision that indemnifies volunteers, including inspectors, against
liability and expenses, including attorney fees, incurred in connection with any legal action in
which the individual is made a defendant by reason of the individual's good faith efforts on
behalf of the CAP. Inspectors approached in this regard by a laboratory, patient, or an attorney
regarding inspection activities should contact the CAP immediately to invoke this provision.
Inspectors may not discuss any inspection findings with anyone outside the inspected laboratory
or the CAP.
Conflict of Interest
Accreditation must be carried out in an impartial and objective manner, uninfluenced by any
personal, financial, or professional interest of any individual acting on behalf of the CAP.
Inspectors must not be engaged in close personal, family, business, or professional
relationships with any personnel in a laboratory that they inspect. An inspector must not solicit or
accept gifts of any type, including personal gifts, products, services, or entertainment. Neither
shall inspectors discuss, solicit, accept, or have an employment or consulting arrangement,
referral of business, or other business opportunity with the laboratory that they inspect.
Laboratories may challenge any deficiency citation. The CAP believes that team leaders and
inspectors will conduct inspections objectively and professionally, regardless of whether they
are in competition with the subject institution. Prior to conducting inspections, the CAP requires
team leaders to sign a statement attesting to the absence of conflict of interest.
The laboratory is notified in advance of the team leader’s name and institution. The laboratory
may contact the inspector if logistical considerations need discussion with the exception of
the actual inspection date when an unannounced inspection is required. Prior to the
inspection, the laboratory may discuss the specifics of a perceived conflict of interest with CAP
staff or the state and/or regional commissioner, or complete and return the conflict of interest
form that is found in the self-inspection materials. CAP headquarters will evaluate and discuss
this information with the state or regional commissioners for final determination. All state or
regional commissioners have discretion to recommend reassignment if there appears to be a
valid conflict of interest.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
16
APPLYING TO CAP ACCREDITATION PROGRAMS
TOPIC
PAGE
Accreditation
Application Process 16
Proficiency
Testing (PT) Prerequisite 17
Application
and Supplemental Materials 17
Laboratory
Disciplines 18
Activity
Menu 19
Research
or Non-CLIA Testing/Procedures 19
Reapplication
Process 19
AABB
Coordinated Inspection 20
Accreditation Application Process
Laboratories seeking accreditation by the CAP must submit an Accreditation Program
Application Request Form along with a nonrefundable application fee. The CAP will send a
Welcome Kit letter via email once the application request is processed. The Welcome Kit letter
will include instructions for:
• Creating an online account
• Accessing the application
• Scheduling an onboarding telephone conference
• Accessing links to several other resources, such as the Master Accreditation Checklists to
help the laboratory prepare for the inspection.
Laboratories seeking first-time accreditation typically complete the first inspection cycle in one
year once an application request is submitted. Timing of events:
• Year 1: Weeks 1-12:
o Submit application request
o Review welcome kit
o Complete application.
• Year 1: Weeks 13-24:
o CAP application review
o Receive customized checklists
o Schedule inspection date
o Host inspection day.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
17
• Year 1: Weeks 25-34
o Submit evidence of corrections to any cited deficiencies.
• Year 1: Weeks 35-52
o Receive certificate of accreditation.
• Year 2:
o Perform self-inspection
o Maintain continuous compliance.
Laboratories with separate CLIA numbers seeking CAP accreditation are accredited separately,
even when operating within the same institution. Laboratories under separate CLIA numbers
seeking CAP accreditation at the same address must have separate CAP numbers, and
likewise must enroll in separate proficiency testing (PT) products. Laboratories operating under
separate CLIA certificates must submit separate fees and application request forms. If a
laboratory chooses to have its inspections coordinated with an existing CAP-accredited
laboratory, this information must be provided in the application.
Proficiency Testing (PT) Prerequisite
• Laboratories applying for accreditation must accurately report each patient-
reportable test that it performs to the CAP.
• For analytes that require external proficiency testing (PT), each laboratory must enroll
and participate in a CAP-accepted PT program. Information on PT enrollment and CAP-
accepted PT programs are found in the
Proficiency Testing: Enrollment and Handling
section.
• For tests that do not require enrollment in a CAP-accepted PT program, the laboratory
must perform an alternative performance assessment semiannually to determine the
reliability of testing. The most common way to do this is by purchasing an external PT
product. Other acceptable alternative performance assessment procedures are listed in
the
Proficiency Testing: Enrollment and Handling section.
• International laboratories are required to enroll in CAP PT for all test/activities if a CAP
PT program is available.
For international laboratories not subject to US regulations seeking CAP accreditation,
enrollment in a CAP Proficiency Testing program is required for a minimum of six months prior
to requesting an Accreditation Program Application Request Form.
Application and Supplemental Materials – New Laboratories
Each laboratory must complete the application by accessing the Organization Profile through e-
LAB Solutions Suite. All required fields are indicated with a red asterisk (*). Short video tutorials
are available on each page of the Organization Profile.
• Required documents to be uploaded include:
o CLIA Certificate (laboratories subject to US regulations) or CLIP certificate (United
States Department of Defense laboratories)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
18
o Previous inspection report (if applicable)
o Laboratory Director CV
o Additional required laboratory director documents, as applicable
Copy of current state medical license
State laboratory director license
Board certification
Equivalency evaluation
o Organization chart
o Litigation Packet (FDT only).
NOTE: Laboratories applying for Forensic Drug Testing (FDT) Program accreditation must
submit the following “litigation packet” information:
• A copy of the overall chain-of-custody (COC) procedure with a flow chart illustrating the
various steps used by the laboratory to ensure specimen integrity from the initial receipt
of a specimen to its final disposition
• Provide a recent (past 30 days) example of a positive THC-COOH data pack in a
litigation format, including:
o Standard operating procedure (SOP) for the screening procedure
o Screening data for the specimens, calibrator(s), and controls
o Evidence of review of the screening batch
o SOP for the confirmation procedure
o Chromatographic data for the specimens, calibrator(s), and controls
o Determination of ion ratios
o Evidence of review
o Copy of the final report (identity of person tested should be redacted)
o Copies of specimen and aliquot internal COC documents.
• Complete Organization Profile Sections:
o Demographics
o General Information
o Relationships
o Roles/Personnel
o Sections/Departments
o Inspections.
Laboratory Disciplines
A discipline is a CAP-defined term used to describe testing or services grouped within a major
category of clinical laboratory science. All disciplines practiced by the laboratory must be listed
in the application, and all disciplines will be inspected. The CAP does not accredit portions of
laboratories.
CAP disciplines/subdisciplines and CMS specialties/subspecialties will be determined by the
selection of activities from the Master Activity Menu. The accreditation letter lists only the
disciplines that are reviewed at the time of the inspection. Laboratories that add disciplines
and/or analytes after the inspection must update their Activity Menu online in the Organization

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
19
Profile. In some cases, additional inspections for added disciplines may be required. (Refer to
the Non-routine Inspections
section of this manual.)
Activity Menu
The laboratory provides information about its scope of testing and lists all reportable assays and
applicable method/scope codes through its Activity Menu. The information provided is used for
the following purposes:
• Customizing checklists
• Determining disciplines for which accreditation is granted
• Verifying and monitoring proficiency testing enrollment
• Determining whether inspectors with specialty training are required
• Determining the laboratory’s annual fee.
Inaccuracies in the Activity Menu may result in a non-routine inspection and additional fees.
Research or Non-CLIA Testing/Procedures
Laboratories are not required to include testing performed solely for the purpose of
research use on activity menus, but may opt to include such testing if the laboratory wants
it to be inspected by the CAP.
• Testing performed for research is defined as laboratory testing on human specimens
where patient-specific results are not reported for the diagnosis, prevention, or
treatment of any disease or impairment of, or the assessment of the health of
individual patients.
• If patient-specific results are reported from the laboratory, the testing is subject to
CLIA and must be inspected by the CAP.
For non-CLIA testing (eg, forensic or pre-employment drug testing) and procedures (eg,
embryology, biorepository specimen processing), all tests and procedures must be
included on a laboratory’s activity menu unless they are distinctly delineated as separate
from the laboratory (eg, separate ownership, separate workspace, separate policies and
procedures).
Reapplication Process
Every two years the laboratory enters a reapplication phase at which time all data and
attachments are reviewed by the laboratory and updated, if necessary. Laboratories should
update data often to keep all information current. Individuals in key roles should maintain their
profile information (eg, email address, phone number) at MyProfile on cap.org.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
20
AABB Coordinated Inspection
Laboratories wanting a CAP/AABB coordinated inspection of their transfusion medicine service
must add an AABB relationship under Licensure and Certification in the Relationship
Section of Organization Profile. Laboratories will need to request CAP/AABB coordination as
part of their Accreditation Application. Additionally, these laboratories must notify the AABB
national office at 301-907-6977 as early as possible in the application/reapplication process to
allow sufficient time for administrative processing. Due to differences in the timing of CAP and
AABB inspection cycles, a coordinated inspection may not be possible for an initial inspection.
CAP will alert a laboratory when coordination is not possible for an initial inspection and will
work with the laboratory to assist with planning for the next inspection cycle.
NOTE: A coordinated inspection with the AABB assessor is pertinent only to the laboratory that
has dual CAP/AABB accreditation. There may be other laboratories in the system providing
transfusion services that are CAP-accredited but not AABB-accredited. These must be
inspected by member(s) of the CAP system inspection team. For questions on preparing for the
inspection or at the time of the inspection, call 800-323-4040 ext. 6065 or 847-832-7000 to
consult with a CAP technical specialist.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
21
INSPECTION OPTIONS
TOPIC
PAGE
Inspection Methods
21
Affiliated Laboratories
21
CAP
Staff Inspections 22
System Inspection Option
23
System
Inspection Eligibility Criteria 23
System Pathologist Role
24
System
Inspection Team Preparation 24
System
Inspection Inspector Tools 25
System
Summation Conferences and the Global Summation 25
Inspection Methods
The CAP offers options to meet the needs of both laboratories and inspectors to complete the
inspection process. These options ensure a safe, collegial, and educational inspection for all
through its options. Use of technology such as document sharing, live streaming, and video
conferencing support the inspection method. All options enable laboratories to be inspected and
remain accredited using the inspection options best suited for their laboratory. The virtual
inspection is an option for laboratories in good standing seeking reaccreditation. All options
provide opportunities to continue the shared experience and exchange of ideas that foster the
connection and pursuit of learning that are hallmarks of CAP inspections.
To meet each laboratory’s unique needs, inspection methods may include:
• In-person or virtual laboratory inspections
• Document review before or during the inspection
• A blend of these options.
Affiliated Laboratories
Affiliated laboratories located within 15 miles or 30 minutes driving distance from the primary
laboratory may have coordinated inspections by the same inspection team. Laboratories that
do not meet the distance criteria may be assigned to another inspection team or a CAP staff
inspector (refer to the CAP Staff Inspection
section below).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
22
Affiliated laboratories are located at physically separate sites but are connected to
another laboratory by management and/or ownership.
• Each site is evaluated as a separate laboratory and has its own CAP number and
CLIA number (if applicable).
• Each laboratory has separate:
o Application materials
o Customized checklists
o Proficiency testing
o Inspections
o Post-inspection review and accreditation decisions
o Accreditation certificates
o Inspection fees.
• Affiliated laboratories may request their own summation conference.
• Each grouping of affiliated laboratories designates a primary laboratory for
communication with the CAP (eg, blackout dates) and the team leader.
• Examples of affiliated laboratories include:
o Two or more hospitals that provide some services at each site (one often
designated as full service and the other as a core laboratory)
o A large commercial laboratory that has branches in different geographic
locations
o A hospital and on-site or remote limited service or special function laboratories,
such as blood gas laboratories, oncology clinics, or smaller branch laboratories
that perform limited testing.
Laboratories may identify and maintain affiliated laboratories for inspection on cap.org
in e-LAB
Solutions Suite in Organization Profile by updating the information in the Inspection Unit link
under the Relationships heading. Affiliated laboratories must share the same accreditation
anniversary date as the primary laboratory.
CAP Staff Inspections
The CAP offers an option for laboratories that perform limited testing to be inspected by CAP
staff instead of a volunteer team. This option is in keeping with the CAP’s philosophy of peer
review because it uses CAP-employed medical technologists that have experience performing
and supervising laboratory testing to perform these inspections.
Laboratories participating in this option typically include:
• Affiliated laboratories that are located more than 15 miles or 30 minutes from the main
laboratory
• Hospitals with 150 beds or fewer that perform basic testing only (such as that seen in a
core laboratory). If on-site anatomic pathology services are offered, they must be limited to
frozen sections, specimen accessioning, and/or FNA adequacy assessment to qualify for
this type of inspection.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
23
Laboratories interested in this option may contact the CAP at 800-323-4040 or 847-832-7000
to discuss if this option is appropriate for their laboratory.
System Inspection Option
Laboratory directors of integrated health care delivery networks may benefit from having their
laboratories participate in the CAP System Inspection option. A CAP System Inspection
consists of two or more full-service laboratories and any associated affiliated laboratories
under the same ownership and administration inspected by one team of inspectors over the
course of several days. These laboratories have highly integrated services and must meet
additional specific eligibility requirements outlined below. Laboratories interested in
participating in the CAP’s System Inspection option should review the information on the CAP
website and download, complete, and return the System Inspection Option Application available
on cap.org
. The system inspection application fee should also be remitted with the completed
system inspection application.
System Inspection Eligibility Criteria
Laboratories participating in the system inspection option must:
• Have common administration and ownership
• Be located within three hours travel time (ground transportation) of a system-defined
central location
• Participate in the CAP Laboratory Accreditation Program
• Meet the system option eligibility criteria for the degree of integration within the system.
Each individual laboratory within the system must meet at least six of the following eight
eligibility criteria:
• Operate on the same set of administrative policies and procedures
• Report directly to one or more central management teams
• Perform common competency assessment at each site utilizing a system-wide
standardized program
• Participate in a system-wide quality improvement plan
• Use the same QC interpretive standards and guidelines for common instruments
and procedures
• Have an integrated information/central data repository or common laboratory
information system (LIS)
• Participate in a common safety program with a common safety manual
• Use a common specimen collection manual.
A CAP inspection specialist schedules and conducts an on-site pre-inspection visit with any
group that is new to the CAP System Inspection option to assess the degree of integration and
develop a plan for the inspection. For existing laboratories participating in the System Inspection

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
24
option, an inspection specialist conducts a pre-inspection conference call with the system
approximately four to six months prior to the laboratory’s anniversary date to reassess the
system’s level of integration of services and plan for the inspection. The information obtained by
the inspection specialist is shared with the team leader and team coordinator to assist with the
inspection planning and team building processes.
System Pathologist Role
A designated system pathologist is responsible for various duties relating to the system’s CAP
accreditation as well as the performance of reciprocal inspections.
System pathologist duties relating to the system’s CAP accreditation include:
• Approving whether a laboratory can join or leave the system
• Ensuring the system planning for an effective and efficient on-site inspection
• Overseeing standardization of activities across all laboratories in the system.
Note that the system pathologist is not responsible for the compliance of each separate laboratory
within the system. The CAP/CLIA laboratory director for each site has the ultimate responsibility for
laboratory compliance (eg, Sign-off on all policies and procedures, approve individualized quality
control plans, carry out laboratory director responsibilities).
System pathologist duties relating to the performance of reciprocal CAP system inspections
include:
• Accepting (or rejecting) a system inspection assignment when contacted by the CAP
• Ensuring the planning for the performance of an effective and efficient system inspection.
Note that the system pathologist is not obligated to take the team leader role for reciprocal
inspections. It may be delegated to another pathologist.
System Inspection Team Preparation
The inspection process is similar to that required to inspect a single laboratory/facility. However,
team size and composition require particular attention and planning. Travel and lodging can be
complex; therefore, use of the CAP Travel Desk staff at 800-323-4040 ext. 7800, is required
for all air travel and hotel accommodations. Once the final team count and inspection dates
have been approved by CAP headquarters, the CAP Travel Desk staff arranges for direct billing
of airfare and lodging and negotiates the best rates for both.
Upon receipt of the inspector’s packet and the pre-inspection report, the team leader will
determine the number of inspectors and days needed to complete the inspection. The CAP
recommends that inspection teams use inspectors who can inspect multiple areas; this
decreases disruption of services at the laboratory and decreases inspection costs. To
assemble the team, the team leader:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
25
• References the Planning Guide for Area(s) of Responsibility and the System Pre-
inspection Information form (refer to the System Inspection Inspector Tools
section
below)
• Include a CAP Inspection Specialist on the team as an active team member. CAP
Inspection Specialists can inspect any clinical checklist, frozen sections, specimen
accessioning, and/or FNA adequacy assessment
• Shares the plans with the assigned CAP Inspection Specialist and inspection
assignment specialist to determine if there is agreement on team size, composition,
time allocation, and the preferred week the inspection will occur.
Inspectors need to prepare for the inspection well before the inspection dates and clarify what
will and will not be inspected. For instance, a system with a central histology/cytology
processing location, but with frozen section and/or interpretive services provided at multiple
locations requires inspection of each laboratory using the relevant portions of the Anatomic
Pathology and/or Cytopathology Checklists.
System Inspection Inspector Tools
One of the goals of a system inspection is continuity in the inspection process. Therefore, the
same inspector should be used to inspect the same discipline in all labs. If this is not possible,
all inspectors inspecting the same discipline must compare their findings between laboratories
before the summation conference to ensure a consistent approach and interpretation of
compliance.
The following supplements are provided in the Systems Inspector’s Inspection Packet:
1. Assessment of System Integration form – Form completed by the system administration
and/or management team at reapplication time that includes information used to assist in
team building. The information can also be included in the global summation conference to
discuss degrees of integration for the system.
2. P
lanning Guide for Inspector Area(s) of Responsibility – Customized Excel
spreadsheet used by the team leader to build the team and ensure adequate inspectors are
used, as well as ensure any specialty inspector needs are met.
3. System Pre-Inspection Information form – Form completed by the system administration
and/or management team before the pre-inspection call/visit and is updated by the
inspection specialist based on discussion with the laboratory. The team leader uses it to
assist in team building.
System Summation Conferences and the Global Summation
A separate summation conference should take place at each laboratory inspected. (Refer to the
Summation Conference section of this manual for detailed instructions related to conducting a
summation conference.)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
26
On the last day of the system inspection (or the following morning if the last day of inspection is
a full day of inspecting), a global summation conference is held that includes:
• A brief presentation for the system personnel being inspected
• A discussion on how the system can further integrate
• System-wide deficiencies and opportunities for improvement
• Areas of excellence and strengths noted during the inspection.
The global summation conference is not intended to be a reiteration of all the deficiencies and
recommendations cited during the inspection of the individual laboratories in the system.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
27
PREPARING FOR THE INSPECTION: LABORATORY
TOPIC
PAGE
Laboratory
Inspection Packet 27
Inspection
Preparation Tips 28
Inspection
Plan 29
Laboratory Inspection Packet
After the laboratory completes its application or reapplication, the CAP sends a Laboratory
Inspection packet with the following documents:
• Cover letter
• Inspection Supplemental Information (inspection blackout dates and hours of operation)
• Activity Menu report
• Checklist Selection report
• Accreditation Checklists (customized).
The checklist edition assigned for an inspection is the current edition available at the time the
application/reapplication is initiated. The checklist edition used for the inspection may be
different than the edition used for the previous or next self-inspection. The cover letter included
in the inspection packet contains the possible dates for inspection and whether the inspection
will be announced or unannounced.
The inspection team receives an inspector inspection packet, which includes the customized
checklists based on the laboratory’s activity menu. Laboratories should carefully review the
activity information to ensure that it is current. The laboratory must update its activity menu
information if there are changes prior to the inspection because changes may impact the
customized checklists. Laboratories should make activity menu changes in Organization Profile
through e-LAB Solutions Suite by logging into cap.org.
The inspection may be in-person or virtual. The laboratory will work with the inspection team
leader to determine the inspection method. Use of technology, such as document sharing, live
streaming, or video conferencing may be used to facilitate the inspection. Refer to the Inspection
Options section for more information.
Laboratories seeking initial CAP accreditation will typically be inspected within 6 months of
receiving the laboratory inspection packet. The inspection team leader will contact the laboratory
to schedule a mutually agreeable inspection date. CAP-accredited laboratories reapplying for
accreditation will be inspected sometime within the 90 days prior to the laboratory’s
anniversary date. The team leader may contact the laboratory to discuss logistics for an
unannounced inspection but may not inform the laboratory of the inspection date.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
28
Inspection Preparation Tips
The following tips may help the laboratory prepare for inspection:
• Prepare references that describe how the laboratory complies with each requirement.
Example:
o Download customized checklists from cap.org
in the Excel format (refer to the
Accreditation Checklists section for information on downloading the checklists in
different formats)
o Add columns to the spreadsheet for comments and/or hyperlinks to policies,
procedures and other compliance documents.
• Refer to the Checklist Changes only version of the checklists available on cap.org
to
identify new requirements and checklist changes from the previous edition.
• Update the Laboratory Personnel Evaluation Roster to include changes in personnel or
supervisor responsibilities.
o Ensure that all CLIA roles relevant to the testing performed by a laboratory are
filled by qualified individuals. For example, a technical consultant must be listed if
any moderate complexity testing is performed. A technical supervisor and general
supervisor must be listed if any high complexity testing is performed.
o Audit personnel records to ensure that all required records are readily available.
Have the updated roster ready to present to the inspection team.
• For laboratories reapplying for accreditation, review inspection findings and records of
corrective actions from the last CAP inspection and the interim self-inspection and
confirm ongoing correction of deficiencies. Ensure that the self-inspection records will be
readily available the day of inspection.
• Review the Activity Menu report to confirm that the laboratory is either
enrolled in CAP-accepted proficiency testing (PT) or performing alternative performance
assessment for each activity/test. Ensure that PT and alternative performance
assessment records are readily available and have been reviewed, with records of
corrective action, as applicable.
• Update the List of Individualized Quality Control Plans (IQCP) form if one or more IQCP
is used in the laboratory. Have the form available to present to the inspection team.
Ensure that related documents supporting the IQCP (eg, risk assessment, quality control
plan, quality assurance monitoring) are readily available.
• De
velop a process for timely retrieval of off-site records, such as personnel training
records and initial instrument/method validation/verification studies. Store on-site
documents and records in a central location so that they are easily accessible during the
inspection. Ensure that relevant staff know how to locate or retrieve the documents and
records.
• Train all personnel to be familiar with the checklists and the inspection process. Ensure
that staff in each laboratory section know where to find specific documents needed for
the inspection.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
29
• Participate in educational activities offered by the CAP to gain a better understanding of
accreditation requirements (refer to Appendix E
, CAP Accreditation Tools and
Resources, for the location on cap.org.
o Focus on Compliance webinar series - CAP accreditation presentations that
focus on key accreditation topics aimed at laboratory professionals. Register for
live events or listen to previously recorded presentations on cap.org.
o Fast Focus on Compliance: Online modules developed to provide information
on a variety of challenging topics in a bite-sized learning format.
Inspection Plan
The laboratory should prepare an inspection plan to avoid confusion and delays on the day of
inspection, including the following elements:
Plan Elements
Example Tasks
One-hour security notification
• Ensure that a responsible person will be available
to receive the one-hour security notification call
from the inspection team
• Define what activities need to occur when the call
is received (eg, notification tree)
Designated central contact
• Designate one or more individual as the central
contact to coordinate events throughout the day
• Greet the inspection team and make introductions
• Arrange for a short laboratory tour at the
beginning of the inspection
List of key personnel
• Identify a list of key personnel who have
knowledge of policies, procedures, and the
location of key documents (eg, QC, PT,
instrument and equipment maintenance and
function checks) for each area of the laboratory
• Include backup personnel in case a contact is not
available on the day of inspection
Interviews with team leader
• Identify representatives from medical staff and
administration who will be available for an
interview with the team leader
• Include backups in case the designated
representative is not available on the day of
inspection
Communication
• Communicate with all parties within and outside of
the laboratory that may be involved in the
inspection process
• Schedule interviews with representatives from the
medical staff and administration

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
30
Meeting rooms and workspace
• Identify options for meeting rooms or workspace to
be used by the inspection team, including a “home
base” in a location convenient to the laboratory
• Identify locations for introductions and for the
summation conference
Transportation to test sites and
facilities
• Establish a mechanism to escort team members
to testing sites
• Provide transportation for off-site locations, if
needed
Staffing needs
• Assess workload and staffing to determine if
modifications are needed to prevent disruption of
patient care
Inspection team needs
• Arrange for refreshments (water/coffee) and lunch
for the inspection team or provide information on
locations for dining located near the laboratory
(provision of refreshments/lunch is optional and at
the discretion of the laboratory)
• Provide personal protective equipment
• Make office supplies available in the team work
area (eg, pads of paper, pens, sticky notes/flags)
• Provide internet and telephone access
Records
• Provide centralized records to be available
throughout the course of the inspection for
policies, procedures, and other records
• Ensure that personnel files are readily available
• Arrange for off-site records needed for the
inspection to be delivered to the laboratory
Conclusion of the inspection
• Provide copying services prior to the summation
conference
• Provide facilities for inspectors to securely dispose
of inspection materials.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
31
PREPARING FOR THE INSPECTION: INSPECTION TEAM
TOPIC
PAGE
Inspection
Team Leader Assignment 31
Team
Leader Qualifications and Responsibilities 32
Inspector’s
Inspection Packet 33
Assembling
the Inspection Team 34
Inspection
Team Member Qualifications and Responsibilities 36
Team
Leader and Team Member Training Options 37
Optional Educational Activities
37
Arranging
the Inspection Date 37
Announced Inspections
38
Unannounced Inspections
38
Arranging
Inspection Team Travel 39
Requests
for Inspection Delays 40
Internal CAP Validation Inspections
40
AABB Coordinated Inspection
40
Inspection Team Leader Assignment
The CAP’s accreditation programs use a peer-based inspection model. CAP-accredited
laboratories are required to provide a trained inspection team comparable in size and scope if
requested by the regional and/or state commissioner, at least once every two-year accreditation
period as a term of accreditation. The assignment is made by matching a team leader from one
laboratory (or group of laboratories) to another laboratory (or group of laboratories) after
screening against multiple criteria, including known conflicts of interest, geographic distance,
and size and complexity of the respective laboratory.
Assignments can be made up to 15 months prior to the anniversary date of the laboratory being
inspected. The team leader receives an inspection assignment letter to confirm an assignment
and report any conflicts of interest. (For information on conflicts of interest, refer to the
Accreditation Program Philosophies
section.)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
32
Team Leader Qualifications and Responsibilities
Team leaders should be:
• A peer of the laboratory director, with similar status, type of practice, and hospital
or laboratory size
• Preferably a board-certified pathologist* and a CAP Fellow
• Affiliated currently or has past experience with a CAP-accredited laboratory
• Trained in the inspection process and in team leader responsibilities
• Not engaged in a close personal, family, business, or professional relationship with any
personnel in a laboratory that he/she will inspect.
* A nonpathologist inspector may serve as the team leader for a laboratory that is typically not
directed by a pathologist (eg, a cytogenetics laboratory) so long as the inspector is a peer of the
laboratory director. For a pathologist-directed laboratory, however, a nonpathologist inspector
may serve as the team leader only with the prior agreement of the laboratory director.
The team leader for a biorepository inspection must have the qualifications to be a laboratory
director of a biorepository.
For anatomic pathology sections, a pathologist, board certified in anatomic pathology, must
perform the inspection, or supervise the inspection if performed by a qualified histotechnologist
or cytotechnologist. One exception is for small laboratories offering anatomic pathology limited
to specimen accessioning, frozen sections, and/or fine needle aspiration adequacy assessment
that are routinely inspected by a CAP staff inspector team. (Refer to the CAP Staff Inspections
section)
Inspection team leaders are responsible for:
• Assembling an inspection team of appropriate size and experience for the laboratory or
laboratories being inspected
• Communicating with the laboratory to determine the inspection method (refer to the
Inspection Options section)
• Ensuring that team members are appropriately qualified and have completed CAP
inspector training
• Setting the inspection date within the correct window
• Making inspection materials available to inspection team members
• Providing overall supervision and time management of the team throughout the
inspection process
• Evaluating compliance with the Director Assessment Checklist, including interviews with
the laboratory director and other institutional representatives
• Conducting the inspection summation conference
• Submitting the post inspection findings and materials to the CAP.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
33
Inspector’s Inspection Packet
The CAP Inspector’s Inspection Packet contains:
• Team leader inspection materials
1. Team Leader Inspection Planner
2. Summary of the laboratories to be inspected
3. Inspection Supplemental Information sheet (days and hours of laboratory
operation, blackout dates for unannounced inspections)
4. Inspection Assignment Worksheet by Laboratory form
5. Travel and Lodging Information form
6. Inspection Team Building Tip Sheet
7. CAP Accreditation Resources for Inspector
8. Team Leader and Team Member training information sheet
9. Standards for Accreditation
10. Prepaid mailer envelope to return the packet to the CAP within 24 hours
after the inspection is completed
11. Team Leader Evaluation form
12. Form to claim Inspection Reimbursement
13. Packet Table of Contents
14. To Cite or Not to Cite guide
15. Name tags for the team (every team member should wear a name tag while in
the host facility)
16. Security clearance forms, if needed.
• Accreditation unit (AU) materials (for each laboratory being inspected)
1. Laboratory Synopsis Report
2. Letter for laboratory director announcing inspection
3. Instructions for Sampling & Evaluating Laboratory Personnel Records
4. Personnel Requirements sheet
5. Laboratory Personnel Evaluation Roster (PER)
6. Complaint Report, if applicable
7. Inspector’s Summation Report (ISR) forms (Part A and “extra copy” pages)
8. Laboratory organization chart
9. Laboratory director’s Curriculum vita (CV)
10. Inspector’s Summation Report from the previous inspection
11. Laboratory-Specific Activity Menu (list of tests and testing modalities).
• Section unit (SU) materials (for each section unit/department)
1. Laboratory Section Synopsis Report
2. Team Member Inspection Planner

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
34
3. Instrumentation list
4. Proficiency Testing Performance Report
5. Team Member Evaluation form.
• Checklist section (separate subsection for each section unit/department)
1. Previous Inspector’s Summation Report (ISR)
2. Laboratory-Specific Activity Menu
3. ISR Deficiency form
4. ISR Recommendation form
5. Customized checklist (based on the laboratory’s activity menu for each section).
• Post Inspection Instructions For Responding to Deficiencies(Blue Folder).
The inspection team should contact the CAP if there are any materials missing or with questions
about the inspection packet.
The CAP provides an electronic inspection packet for volunteer and staff inspectors. The
electronic inspection packet is intended to complement the standard paper packet and make it
easier for a team leader to distribute inspection materials to team members and specialty
inspectors.
Team leaders will receive an email notification with a download link when the electronic
inspector packet is available and each time a change is made that impacts the inspection. The
team leader may also access this information by logging onto cap.org and navigating to the
inspection download through the Inspection Packets for Team Leads link in the Inspector
Training and Resources section on the Laboratory Improvement/Accreditation page.
A team leader can share an electronic packet with the team members. When selecting the share
link in the Action column, a pop-up window opens that includes link to the electronic packet and
allows the team leader to enter a recipient email address.
Assembling the Inspection Team
The team leader should immediately review the materials in the Inspector’s Inspection Packet
upon receipt and begin assembling the inspection team. The packet contains the information on
the appropriate number of inspectors and the expertise needed. It recommends the “number of
inspector days” to perform the inspection, based upon the disciplines and test volumes declared
by the laboratory.
For inspections of large or multisite laboratories, the team leader may decide to spend more
than one day on site with a smaller team, rather than taking a team large enough to complete
the inspection in one day. This approach is helpful when section supervisors are responsible
for more than one site and may not be available at more than one site during a one-day
inspection.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
35
General guidelines for assembling the inspection team:
• One inspector is needed for the Laboratory General inspection. More than one inspector
may be needed for large, full-service laboratories, such as a university hospital
laboratory. Alternatively, inspectors assigned to other checklists may be able to assist
the Laboratory General inspector with sections of the checklist (eg, computer, safety).
• One inspector may be able to inspect with more than one discipline-specific checklist or
inspect more than one laboratory section during an inspection depending on the
experience of the team member, the scope of testing performed, and set up of the
laboratory. Common combinations include:
o Hematology and Urinalysis
o Chemistry and a separate blood gas laboratory
o Microbiology and Immunology – A second inspector may be needed if the
laboratory offers extensive services in microbiology in all subdisciplines
(bacteriology, mycobacteriology, mycology, parasitology, virology, and molecular
microbiology).
o Anatomic Pathology and Cytopathology
o Transfusion Medicine and Immunology – A second inspector may be needed for
hospital laboratories that have extensive donor and transfusion activities.
• Fewer inspectors are needed for laboratories with very limited test menus. One
generalist inspector may be able to inspect using the Limited Service Laboratory
Checklist.
• Adjustments to the number of inspectors should be made based upon the experience of
the inspectors and the extent of testing in the laboratory.
The Inspection Team Building Tip Sheet found in the inspection packet contains additional
information for assembling the team.
The CAP requires the use of a specialty inspector for inspections performed with the following
checklists:
• Cytogenetics
• Flow Cytometry
• Histocompatibility
• Clinical Biochemical Genetics
• Molecular Pathology.
Specialty inspectors will be listed on the Inspector Candidate Roster Report. Potential team
members not on the report may apply to be a specialty inspector on cap.org
by logging in to My
Profile, selecting the “Business/Professional” tab and then “Request to be Inspector” to complete
the data needed for the inspector database. The team leader may bring the potential team
member only if he or she receives approval for that specialty.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
36
If a team leader wants to take more inspectors than the CAP recommended number
provided in the inspector packet, the team leader must contact the CAP prior to the
inspection to obtain approval. Additional inspectors may not be reimbursed without prior
approval. The team leader must:
• Complete the Inspection Assignment Worksheet by Laboratory form included in the
packet and explain why additional inspectors are needed
• Email the form to [email protected].
The CAP will review the request and notify the team leader within two business days about the
approval decision.
Team leaders may obtain assistance to identify additional inspectors to perform the inspection
by contacting the CAP at 800-323-4040, ext. 6061 or 847-832-7000, ext. 6061. The CAP can
provide lists of qualified inspectors from its CAP inspector database.
Inspection Team Member Qualifications and Responsibilities
The team leader assembles the inspection team by selecting team members with the necessary
expertise in the assigned inspection areas. All inspectors must be trained on the inspection
process (refer to the Inspector Team Leader and Team Member Training Options section).
Inspectors may include:
• Experienced medical technologists/clinical laboratory scientists, preferably with
laboratory supervisory or manager experience
• Cytotechnologists
• Histotechnologists
• Doctoral scientists
• Pathology residents and fellows
• Pathologists.
Inspectors must not:
• Inspect a laboratory or facility for which he or she has provided or is likely to
provide consultative services
• Be engaged in close personal, family, business, or professional relationships with
any personnel in a laboratory that the inspector inspects.
Inspection team members must prepare several weeks prior to the inspection to perform a
thorough and efficient inspection.
• Review information provided by the team leader from the inspector’s packet (refer to the
Conducting the Inspection: General Principles and Meetings section)
• Complete inspection team member training and participate in optional educational
activities.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
37
Team Leader and Team Members Training Options
The CAP requires inspectors to successfully complete CAP-approved training prior to
inspecting. Training promotes a more thorough and effective inspection through development of
a consistent understanding of program standards and a uniform application of inspection
techniques. Training is mandatory for all team leaders and team members. Team leaders must
ensure that their team members have fulfilled the training requirement.
Specially designed online training options are available on cap.org
that emphasize the
knowledge and skills required to perform an inspection, including:
• Team Leader Inspection Training
• Team Member Inspection Training.
Completing online inspection training fulfills the training requirement and offers CME/CE credit to
participants. Trained inspectors are encouraged to review the content that is most relevant to
their needs as the training courses are updated annually. In addition to online inspection training,
certain live inspection training events may satisfy the training requirement as well. These events
are specifically designated as such by the CAP.
To enroll and participate in online training, go to cap.org,
click on the Learning Tab, then under
Choose Your Learning Path you will be able to select Learning for Laboratory Professionals,
then Inspector Training – View Courses.
Optional Educational Activities
The CAP offers different educational activities to help inspectors and laboratories stay current.
(Refer to Appendix E, CAP Accreditation Program Website Tools for the location on cap.org
.)
Fast Focus on Compliance: Online modules developed to provide information on a variety of
challenging topics in a bite-sized learning format. Inspectors are encouraged to review these
modules prior to inspecting for the most up-to-date information and inspector tools.
Focus on Compliance webinar series: CAP accreditation presentations that focus on key
accreditation topics aimed at laboratory professionals. Register for live events or listen to
previously recorded events on cap.org
.
Arranging the Inspection Date
The team leader is responsible for arranging the inspection date and notifying the CAP of the
chosen date. Inspections are performed as either announced or unannounced depending on the
type of inspection or laboratory. This information is found in the Team Leader letter in the
inspection packet. The following types of inspections are generally conducted as announced
inspections:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
38
• Laboratories seeking initial CAP accreditation through the Laboratory Accreditation
Program
• Laboratories participating in the Reproductive Laboratory Accreditation Program,
Forensic Drug Testing Accreditation Program, or Biorepository Accreditation Program
• International laboratories.
All other types of inspections are conducted as unannounced inspections.
Announced Inspections
To arrange the inspection date, the team leader must:
• Contact the laboratory director(s) within two weeks of receiving the Inspector’s
Inspection Packet. Contact all directors if special function laboratories are to be
inspected in conjunction with the main clinical laboratory. The inspection date must be
mutually agreeable to all laboratory directors.
• Ensure that the inspection occurs no more than 90 calendar days before the
laboratory’s anniversary date for routine inspections. A mutually acceptable date is
preferable; however, the inspection is scheduled at the convenience of the inspector.
• Send a courtesy letter to the laboratory director(s) or phone call to discuss:
o The inspection date/time
o Inspection Method: in-person or virtual (must meet eligibility criteria)
o Laboratories preference for document review during the inspection, in advance, or
a blend of both
o Projected schedule
o Team listing
o Special requests (eg, histology slides for review) and preliminary instructions
regarding availability of documentation (personnel and training records,
procedure manuals, proficiency testing results, test validation studies, QC and
maintenance records, and a sampling of completed case records [as applicable])
o Key contacts.
• Notify the CAP of the inspection date and the number of inspectors by telephone at 800-
323-4040 or 847-832-7000 or email at accred@cap.org.
Unannounced Inspections
To arrange the inspection date, the team leader must:
• Review Inspection Supplemental Information sheet in the Team Leader materials for
the days and hours of operation and inspection blackout dates
• Ensure that the inspection occurs no more than 90 calendar days before the
laboratory’s accreditation end date of initial accreditation for routine
inspections

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
39
• Notify the CAP of the inspection date and the number of inspectors by telephone at
800-323-4040 or 847-832-7000, or email to
• Consider preparing an inspection schedule that can be handed to the laboratory
director at the beginning of the day. At minimum, this would consist of a list of
inspectors and their section/checklist responsibilities
• For unannounced inspections, the team leader may contact individuals from the
laboratory being inspected to discuss logistics, including inspection method, but must
never inform the laboratory personnel of the inspection date.
Arranging Inspection Team Travel
The CAP will assist the inspection team in meeting its travel needs and requires that all
arrangements be made through the CAP Travel Desk. The travel desk agents can be reached
by:
• Phone: 800-323-4040 ext. 7800 or 847-832-7800, from 8:00
AM–5:00 PM Central Time
• Fax: 847-832-8800
• Email: captraveldesk@cap.org.
When booking travel, the inspection team must provide:
• The five/six-digit Inspection Instance (II) identification number of the laboratory to
be inspected (found on the Laboratory Synopsis page of the inspector packet)
• Inspector names, including gender and birthdates exactly as they appear on the photo
identification used for traveling.
The CAP encourages booking two months prior to travel in order to obtain favorable rates.
When arranging travel, follow the recommended number of inspector days. For requests to
bring additional inspectors beyond the CAP recommended number, contact the CAP at 800-
323-4040 or 847-832-7000. Do not make travel arrangements until the additional inspector
days have been approved.
The CAP Travel Desk agents can also arrange hotel accommodations and rental cars, if
applicable. The CAP Travel Desk can negotiate a master account to cover the room rates and
taxes for inspectors. Inside the US, inspectors should decline insurance for rental cars. Outside
the US, the inspectors should purchase the rental car insurance. Prior to the inspection,
inspectors should contact their personal auto insurer to advise them that they will be driving
outside of the US.
Team members needing to change any travel should contact the CAP Travel Desk agents as
soon as possible.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
40
Requests for Inspection Delays
Council on Accreditation policy requires that laboratories performing patient testing be
prepared for inspection at any time. Any problems encountered in scheduling inspections
should immediately be brought to the attention of the CAP at 800-323-4040 or 847-832-7000
for resolution.
Internal CAP Validation Inspections
The CAP randomly selects a small number of on-site inspections to perform an internal CAP
validation inspection. The assigned inspection team is expected to participate in the internal
CAP validation inspection if selected. The CAP notifies the team leader prior to the inspection if
a validation inspection will occur.
The internal CAP validation inspection is a quality assurance process designed to improve the
consistency and thoroughness of Laboratory Accreditation Program inspections. A CAP staff
inspector is added to the inspection team as an observer for the on-site inspection to gather
information on how the team uses the inspection tools and to identify best practices from the
team that could be added to the inspector training course. The process includes:
• Observation of the volunteer inspection team during the on-site inspection by a CAP
Inspection Specialist
• Inspection of 20 checklist requirements by a CAP Inspection Specialist independent of
the volunteer inspection team and then comparing the results and reconciling any
differences
• Asking the inspectors questions about the inspection process and suggestions for
improvement.
AABB Coordinated Inspection
The CAP has an agreement with the AABB to coordinate the inspections of transfusion services
for laboratories accredited by both the CAP and AABB upon request of the laboratory. The
coordinated inspection may occur on the same or different day than the rest of the laboratory
but must occur before the CAP anniversary date. The following process is followed for AABB
coordinated inspections:
• When the CAP receives notification from the AABB that an AABB assessor has been
assigned, the CAP sends a notification to the team leader, the AABB assessor, and the
laboratory director, providing contact information and defining each inspector’s
responsibilities. The CAP team leader and AABB assessor should work together to
determine if a coordinated inspection can occur on the same day.
• The CAP will send the AABB assessor a packet containing the Transfusion Medicine,
Laboratory General and All Common Checklists, an Inspector’s Summation Report
(ISR) form, the laboratory director’s CV, an organizational chart, a Personnel Roster
(PER), instructions, a letter informing the assessor of the name and telephone number

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
41
of the CAP team leader, and a return envelope.
• The AABB assessor will notify the CAP of the inspection date.
• After the AABB inspection, the AABB assessor completes the CAP Transfusion
Medicine inspection report, leaves a copy with the laboratory, and returns the original
to the CAP in the envelope provided.
The CAP team leader should not hold his/her report to await the AABB assessor’s report. The
CAP accreditation decision will occur only when inspector findings from both organizations have
been submitted to the CAP.
Each organization (the CAP and AABB) makes separate accreditation decisions, and one
organization’s decision does not affect the other.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
42
PROFICIENCY TESTING: ENROLLMENT AND HANDLING
TOPIC
PAGE
Proficiency Testing Enrollment
42
CAP
-Accepted Proficiency Testing Programs 43
Proficiency
Testing Enrollment for Multiple Matrices 43
Proficiency
Testing Handling 44
Proficiency Testing Enrollment
Each accredited laboratory must enroll and participate in a CAP-accepted proficiency testing
(PT) program for all analytes that require PT to assess the accuracy of testing performed. In
some countries, proficiency testing may be referred to as an external quality assessment (EQA)
program. Laboratories should place orders with the PT provider by December 1
st
each year to
ensure full participation.
The following tools can be used to determine which analytes require enrollment in a CAP-
accepted PT program:
• Master Activity Menu with PT Options report available through the e-LAB Solutions Suite
customer portal on the CAP website (
cap.org)
• CAP Surveys catalog - Analyte/Procedure Index section (available on cap.org).
The CAP accreditation programs do not typically require PT enrollment for calculated analytes.
However, there are a few exceptions where PT enrollment is required (eg, estimated
hemoglobin, calculated hematocrit, and non-waived INR calculation.
International laboratories are required to enroll in CAP PT for all test/activities that have an
available CAP PT program.
Laboratories subject to US regulations must participate in one CAP-accepted PT program for
each required analyte for one year before designating a different CAP-accepted PT program
for PT compliance and reporting to the Centers for Medicare and Medicaid Services (CMS). If a
laboratory enrolls in PT mid-year due to a new application for accreditation or the introduction
of new testing, the laboratory may change to another CAP-accepted PT provider at the next
enrollment period without waiting a full year. If a laboratory enrolls in PT from multiple PT
providers for one required analyte, it must designate only one PT provider to submit a
performance score to the CMS for the year.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
43
Alternative performance assessment (APA) must be performed at least semi-annually for tests
for which CAP does not require enrollment in PT. The laboratory director must define such
APA procedures. The criteria for APA must be in accordance with good clinical and scientific
laboratory practice. The laboratory must evaluate each unacceptable PT and each APA result
that does not meet the laboratory’s acceptability criteria. Examples of APA include (in order of
preference):
• Participation in an external PT program supplied by the CAP
• Participation in an ungraded/educational PT program
• Split-sample analysis with referral or other laboratories
• Split-sample analysis with an established in-house method, assayed material, or
regional pools
• Clinical validation by chart review, or other suitable and documented means.
CAP-Accepted Proficiency Testing Programs
The CAP accreditation programs have defined criteria for CAP-accepted PT programs and
analytes. Each PT provider maintains its own list of accepted analytes. Not all analytes within a
PT provider’s program are necessarily accepted by the CAP accreditation programs. It is the
responsibility of accreditation participants to verify analyte acceptance with their PT provider.
NOTE: International laboratories not subject to US regulations seeking CAP accreditation, must
enroll in the CAP PT Program for a minimum of six months prior to initiating the CAP
application process. Laboratories may use acceptable alternatives when the CAP is unable to
deliver PT due to oversubscribed programs, stability issues, or customs denial, contingent on
CAP approval.
Proficiency Testing Enrollment for Multiple Matrices
PT may be required for both serum/plasma and whole blood matrices for some tests. If not
required, laboratories may either:
• Enroll only in the PT program for the laboratory’s primary sample matrix and perform
APA for the other matrix
• Enroll in separate PT programs for both matrices.
Urine and body fluids are unique matrices. Calibrators, reagents, reference ranges and/or
clinical decision-making values usually differ from those for serum, plasma, or whole blood.
The laboratory must:
• Enroll in a PT program specific for that matrix if a CAP-accepted PT program is required
• Perform APA if a CAP-accepted PT program is not required.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
44
Proficiency Testing Handling
Among the requirements of the Clinical Laboratory Improvement Amendments (CLIA)
regulations (section 493.801) is that the laboratory must test PT samples in the same manner
as it tests patient specimens. This means that:
• PT samples should be tested along with the laboratory’s regular workload by personnel
who routinely perform the testing (eg, if a laboratory tests each patient specimen only
once, PT specimens must also be tested only once).
• PT samples should be rotated over time among all staff members and all shifts that
routinely perform the patient testing.
The CLIA regulations also specify the following:
• Laboratories may not engage in any inter-laboratory communications pertaining to the
results of PT samples until after the deadline for result submission to the PT provider
has elapsed.
• Organizations that have laboratories at different test sites with different CAP/CLIA numbers
must prevent such inter-laboratory communication.
• A laboratory may not refer any PT material for testing to another laboratory and may not
accept PT material from a laboratory with a different CLIA/CAP number).
A laboratory must only report results for PT performed in its own laboratory location. It may not
refer PT specimens to another laboratory and report those results. Here are examples of
inappropriate PT referral:
• A laboratory’s routine process for patient testing is to perform only preliminary testing
and to refer the specimen to another laboratory for confirmatory testing. Staff referred PT
samples to another laboratory for confirmation in error. (The laboratory should have
reported the preliminary result to the PT provider and not referred the specimen for
confirmatory testing.)
• A satellite laboratory’s procedure requires abnormal blood smears to be reviewed by a
pathologist at the main laboratory prior to reporting. Staff at the satellite laboratory sent
abnormal blood smears from the PT to the main laboratory in error. (The laboratory
should have submitted a PT response indicating that the test is not performed on-site
and would refer to another laboratory.)
• A main laboratory has all PT kits for its satellite laboratories shipped directly to the main
laboratory. The main laboratory accidentally forwarded a kit to the wrong satellite
laboratory. The satellite laboratory then reported its results under the wrong CAP/CLIA
number.
The laboratory director must ensure that there is a well-established process for the handling of
PT materials, including circumstances that could be considered PT referral. The penalty for
violating PT referral regulations, according to the CMS, may be “revocation of the laboratory’s
CLIA certification for at least one year” and the potential prohibition of the owner or laboratory

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
45
director to own or direct a laboratory for two years. The CAP accreditation programs may
impose additional sanctions including loss of accreditation.
The sole exemption to the “no referral” rule is those laboratories that send slides to another
facility for immunohistochemistry (IHC) staining but perform the interpretation in-house. Staining
is not considered a test. In that case, the IHC staining (and only the staining) of the PT slides
may be referred to the usual outside facility.
Other methods often used in distributive testing such as in situ hybridization, next-generation
sequencing, and flow cytometry are classified as testing under CLIA; therefore, proficiency
testing samples for this testing may not be referred to another laboratory.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
46
PROFICIENCY TESTING: FAILURES AND MONITORING
TOPIC
PAGE
Evaluation of
Proficiency Testing (PT) and Alternative Performance
Assessment (APA)
46
Proficiency
Testing Monitoring 47
Proficiency Testing Compliance Notice (PTCN) – Non-enrollment
47
Proficiency Testing Compliance Notice (PTCN) – Non-participation
48
Proficiency Testing Compliance Notice (PTCN) – Performance
49
Cease Testing Requirement
49
Evaluation of Proficiency Testing (PT) and Alternative Performance Assessment (APA)
Laboratories must review the reports from their PT providers for each PT event and their APA
results to evaluate their performance, investigate each unacceptable result, and take
appropriate corrective action. All records for the event must be retained, including original
worksheets and follow-up investigations.
When investigating PT or APA failures and biases, the following actions may be necessary:
• Check reporting forms and records of sample preparation and testing for non-analytic
(eg, clerical) and analytic errors
• Review QC performance, instrument calibration, and reagent performance prior to,
during, and after the time of PT failure
• Verify that the PT material was stored, handled, and processed according to the kit
instructions (eg, test in correct instrument mode, correct units of measure).
• Investigate biases or trends (as defined in the lab’s policy on PT review)
• Contact the instrument/reagent manufacturer for assistance
• Repeat the PT challenge, if possible, using a different reagent lot or instrumentation
system
• Confirm that patient/client results were not affected during the period of time the PT was
unacceptable.
The laboratory must evaluate and retain records of investigation for each unacceptable PT or
APA result. Corrective actions that may be taken include:
• Repeat instrument function or testing system verification
• Modify the frequency of calibration
• Re
vise or replace the analytic procedure
• Design a process to double check clerical entries prior to submitting PT results

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
47
• Ensure all staff know when PT kits are scheduled to arrive and due dates for PT results
submission
• Retrain testing personnel in the proper procedures for sample preparation, testing, and
reporting.
Occasionally PT challenges are not graded. The CAP accreditation programs require
laboratories to perform a self-evaluation when a PT result is not graded. The laboratory director
is responsible for choosing a self-evaluation method appropriate for the laboratory’s individual
circumstances. Any PT results falling outside the laboratory’s established criteria for acceptable
performance must be investigated and corrective action taken, as would be done for graded PT
results.
Proficiency Testing Monitoring
The CAP monitors three PT compliance processes:
• Confirmation of enrollment in required PT
• Participation in required PT
• Successful PT performance.
Laboratories will receive a Proficiency Testing Compliance Notice (PTCN) for failure to enroll in
PT, participate in PT, or for unsatisfactory performance. The PTCN contains instructions
regarding the actions that must be taken. The CAP Accreditation Programs’ PT Compliance
Department evaluates the actions taken to ensure that the underlying compliance issue is
corrected, and the testing is performed in a manner that will not jeopardize patient safety.
A description of each type of PTCN follows. For help or more information on PT enrollment or
assistance with troubleshooting PT failures, accreditation participants may:
• Refer to the Proficiency Testing/External Quality Assurance Toolbox available through
the e-LAB Solutions Suite customer portal on cap.org
• Contact the CAP at 800-323-4040 or 847-832-7000.
For ideas on troubleshooting analytical issues, participants may refer to the Clinical and
Laboratory Standards Institute Guideline QMS24-ED3, “Using Proficiency Testing and
Alternative Assessment to Improve Medical Laboratory Quality [2016].”
Proficiency Testing Compliance Notice (PTCN) – Non-enrollment
The CAP will send a PTCN if a laboratory is not enrolled in PT for a required analyte that is
listed on its Laboratory-Specific Activity Menu. Enrollment is monitored on a continuous basis.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
48
To respond to the PTCN, the laboratory must take one of the following actions:
• Enroll in the appropriate PT
• Submit payment. Orders cannot be shipped until payment is received
• Provide import permits or certificates of origin, if applicable. Orders cannot be shipped
until all permits are received by the CAP.
• Delete activity from the Laboratory-Specific Activity Menu if the test is no longer
performed
• Contact the PT provider to send enrollment data to the CAP if enrolled with a CAP-
accepted PT provider other than the CAP
• Respond to the CAP with supporting documentation that the intended, CAP-accepted,
PT program is oversubscribed or otherwise unavailable. The laboratory must implement
an APA for the affected analyte(s) using, at minimum, the same number of challenges
as the event missed. For regulated analytes, if the CAP and CAP-accepted PT
programs are oversubscribed, the CMS requires the laboratory to attempt to enroll in
another CMS-approved PT program.
Proficiency Testing Compliance Notice (PTCN) – Non-participation
The CAP monitors participation in PT for each testing event, looking at all analytes that require
PT according to the Laboratory-Specific Activity Menu. A PTCN for non-participation is sent to a
laboratory when it is enrolled in PT for a particular analyte, but the CAP accreditation programs
did not receive PT scores for that analyte. This is due to a failure to submit results to the PT
provider. This can occur for several reasons, such as:
• A d
iscontinued test was not removed from the Laboratory-Specific Activity Menu.
• Results were transmitted improperly.
• The PT kit was not received by the laboratory.
• PT results were submitted after the due date.
NOTE: Under both CLIA and CAP requirements, failure to participate in a testing event or failure
to return results by the due date is equivalent to a zero score (0%) for the testing event and is
considered unsatisfactory performance.
A laboratory must respond to every non-participation PTCN. The response to the CAP is to
include:
• Reason results were not reported
• Evidence of alternative assessment (if appropriate)
• Records of corrective action taken to prevent recurrence of the error.
If PT was performed, but results were not reported, the laboratory may self-evaluate its own
performance on the event by comparing the laboratory’s results to the statistics in the
Participant Summary Report and use that as evidence of APA. If other means of APA is used,
it should be performed to the same extent as the missed event (eg, number of challenges).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
49
Proficiency Testing Compliance Notice (PTCN) – Performance
PT performance monitoring is a process that looks for instances of unsatisfactory performance
continuously across all testing events. If the performance of an analyte or subspecialty falls
below the acceptable criteria, a PTCN for performance is sent to the laboratory and the
laboratory must respond to the CAP as directed. The laboratory must investigate each
unacceptable PT result and record the investigation to include:
• Reason for the PT failure
• Evidence of how the laboratory ensured the accuracy of patient results
• Specific corrective action taken to prevent recurrence of the problem
• Evidence of APA (if appropriate).
First time PT failures for most analytes or subspecialties (other than predictive markers) do not
require a response to the CAP. Investigate the reason for each PT failure. The inspector will
review and evaluate records of the investigation and corrective action during the next
inspection.
Subsequent PT failures for the same analyte or subspecialty results in unsuccessful PT
performance, the laboratory must complete the PTCN response form, including the reason for
the first failure and provide records of corrective action to the CAP. The laboratory must retain
copies of the correspondence and corrective action.
CAP PT Compliance technical staff will request additional information if the response is
incomplete. CAP staff may also provide informational letters with recommendations to assist
the laboratory with improving its current testing processes for the analyte or subspecialty in
question.
C
ease Testing Requirement
The Clinical Laboratory Improvement Amendments of 1988 (CLIA) mandate that if a laboratory
has repeat unsuccessful performance in PT for a CLIA-regulated analyte, test, subspecialty, or
specialty, the laboratory will be directed to cease testing for six months. As an accrediting
organization deemed by the Centers for Medicare and Medicaid Services (CMS), the CAP has
been directed to enforce this requirement.
Repeat unsuccessful PT performance occurs when the laboratory incurs either three consecutive
or two sets of two out of three unsatisfactory scores over the most recent 12 PT events, for a
CLIA regulated analyte, subspecialty, or specialty. This is defined based on current policies and
guidance from CMS and information available in the CLIA PT monitoring system.
A laboratory that has repeat critical performance for a non-regulated analyte/test may also be
directed to cease testing for an extended period of time (not subject to the six-month period
stipulated for CLIA-regulated analytes/tests).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
50
Before the laboratory can resume testing, it is required to:
• Submit an acceptable plan of corrective action to the CAP
• Provide evidence on how the laboratory ensured the accuracy of patient results
• Demonstrate minimum acceptable performance on reinstatement PT
o At least 80% on reinstatement PT for a regulated analyte/subspecialty/specialty
o 100% for ABO, Rh, and compatibility testing.
If the laboratory refuses to cease testing when directed, its accreditation will be in jeopardy. The
inspection team will verify that laboratories have ceased patient/client testing, if directed by the
CAP, during the next inspection.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
51
ACCREDITATION CHECKLISTS
TOPIC
PAGE
Accreditation
Checklists 51
Checklist
Components 52
Requirement
Components 52
Checklist
Customization 53
Downloading
Checklists from cap.org 54
Identifying
Checklist Changes 55
Assistance
with Checklist Interpretation 55
Accreditation Checklists
Each checklist contains a detailed list of requirements used by laboratories for inspection
preparation and by inspectors to assess compliance. The full set of checklists includes
approximately 2,800 requirements in 21 different checklists organized around specific laboratory
disciplines and/or important management operations. (Appendix A
: Accreditation Checklists
Overview)
The checklists are revised periodically (usually once a year) based on input from CAP’s
practicing experts, such as its scientific committees, inspectors, and participants. The edition of
the checklists chosen for an inspection is the edition sent at the time of
application/reapplication, even if a newer edition has been published. The checklist edition
used for the inspection may be different than the edition used for the previous or next
self-inspection.
The Checklists are copyrighted works of the College of American Pathologists (CAP). The CAP
has authorized copying and use of the checklists by CAP inspectors in conducting laboratory
inspections for the CAP’s accreditation programs and by laboratories that are preparing for
such inspections. Laboratories may upload their electronic checklist into a secure software
system used for purposes of managing accreditation compliance. Laboratories may not provide
the accreditation checklists to software providers for broader distribution.
Except as permitted by section 107 of the Copyright Act, 17 section 107, any other unauthorized
use or distribution of the Checklists constitutes infringement of the CAP’s copyrights in the
Checklists. The CAP will take appropriate legal action to protect these copyrights. Individuals
seeking to use the Checklists for other purposes must contact the CAP to request permission at
checklistcomments@cap.org
.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
52
Checklist Components
The Checklists define the accreditation program requirements. Additional language is often
added to explain a requirement, or to streamline the inspection process. This section describes
the different elements that make up the checklist:
• Table of Contents: List of the headings of each checklist in the order in which they
appear.
• Summary of Checklist Edition Changes: List of new, revised, and
deleted/moved/merged requirement numbers in each checklist.
• Introduction: Information included in the beginning of the checklist or a section of the
checklist to help orient users to that checklist or section.
• Definition of Terms: A glossary of commonly used terms may be found in the
Laboratory General, All Common, Director Assessment, and Biorepository Checklists.
• Inspector Instructions: Read-Observe-Ask-Discover (ROAD): An inspection tool that
shows the inspector how to assess compliance through focusing on a group of related
requirements rather than assessing each requirement individually. The ROAD
instructions appear in the checklist version sent to inspectors and are also available in
the Master versions of each checklist.
• Requirements: Specific elements that CAP-accreditation participants must comply with
to be eligible for accreditation.
Requirement Components
Every requirement includes a Requirement Number, Subject Header, Phase, and Declarative
Statement. Many requirements also add a NOTE, Evidence of Compliance, and/or References.
Example Requirement

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
53
1. Requirement Number: Unique identifier assigned for each requirement made up
of a three-letter checklist abbreviation followed by a five-number code (eg,
GEN.23584).
2. Subject Header: Key words that identify the content of the requirement.
3. Phase: Designation used by the CAP’s accreditation program to differentiate
deficiencies based on the potential impact to the quality of services and the
actions required when cited as a deficiency. The following chart summarizes the
differences between Phase I and II deficiencies:
PHASE
DESCRIPTION
Phase II
• Requirements for items that may seriously impact the
quality of services, endanger patients, clients, or
personnel, or impact regulatory compliance
• Citations require a written response of compliance and
supporting documentation prior to accreditation
Phase I
• Requirements for items that may compromise the
quality of services, but not endanger patients, clients, or
personnel
• Citations require a written response to the CAP
indicating corrective actions, but do not require
supporting documentation unless specially requested
by the CAP
4. Declarative Statement: One or more sentences that define elements required for
compliance.
5. NOTE: Information that provides additional details to assist in interpreting the
requirement. Information in the NOTE is considered integral to the requirement
and must be complied with as part of the declarative statement itself, unless it is
expressed as a best practice or a recommendation.
6. Evidence of Compliance: List of suggested ways to demonstrate compliance
with the requirement (eg, policies, procedures, records, reports).
7. References: Resources, such as peer-reviewed journals, regulations, professional
guidelines, and textbooks that may be helpful.
Checklist Customization
No two laboratory sections or departments are the same. The CAP customizes participants’
checklists for their inspections. Customized checklists link activities (eg, tests, scopes of
service, methods) reported by each section of the laboratory to the applicable checklist
requirements.
College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
54
To ensure proper customization of a checklist, participants must:
• Carefully complete application materials for the activity menu and
• Update the activity menu on cap.org
whenever activity menu changes occur.
Information on completing the activity menu or changing the activity menu can be found in the
sections on Applying to CAP Accreditation Programs and Maintaining Accreditation
.
Downloading Checklists from cap.org
To stay current with changes to the checklist, the CAP encourages participants to download and
review checklists at any time from the e-Lab Solutions Suite customer portal on the CAP
website (cap.org
). The website versions contain elements that are not found in the print versions
mailed to accreditation participants that may be helpful, such as the References and Inspector
Instructions (ROAD).
Accreditation participants have the following dropdown options through the portal:
• Section/Department – identify the area of interest
• Checklist Module – choose from different checklists used in that area
• Checklist Edition – select either the current, published checklist version or checklists that
will be used during inspection or self-inspection
• Checklist Type - select the Master, Custom, or Changes Only
• Checklist Format - choose from PDF, Word/XML, and Excel formats.
The following table describes the different checklist types available:
Type
Features
Availability
Master
• Contains all requirements in
the specified checklist
•
Includes references
•
Useful when starting new types
of testing or services
• Accessed via cap.org (log-
in required)
•
Available for purchase by
non-participants in CAP
accreditation
Custom
• Based upon each section
unit/department’s activity
menu
•
Includes only
those requirements that pertain
to the testing or services
offered
•
Focuses attention on
applicable requirements
• Accessed via cap.org (log-in
required)
•
Mailed to accreditation program
participants for inspection
preparation and performance of
interim self-inspections
•
Included in the inspection
packet materials provided
inspection team leaders
•
Access via cap.org (log-in
required)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
55
Changes
Only
• Contains only those
requirements that have been
changed, added, or deleted
since the previous edition
•
Quickly identifies changes to
requirements in a track
changes format
• Access via cap.org (log-in
required)
Identifying Checklist Changes
There are four ways to identify checklist edition changes.
• Summary of Checklist Edition Changes: Following the Table of Contents in each
checklist there is a listing of new, revised, and deleted requirement numbers. These
requirements remain on the list for 18 months.
• NEW and REVISED Flags: Each new or significantly revised requirement is marked
directly in the checklist with a “NEW” or “REVISED” flag and the date of the edition in
which the requirement first appeared or was changed. The flags remain for 18 months.
• Changes Only Checklist: This checklist type may be downloaded from cap.org
. It
shows what has been changed, added, or deleted since the previous edition in a track
changes format (log-in required).
• Focus on Compliance Webinar Series: The annual “Checklists Update” webinar
provides information on the principal changes to each edition. It may be accessed by
logging into the e-Lab Solutions Suite customer portal on the CAP website at cap.org
and searching: Focus on Compliance.
Assistance with Checklist Interpretation
For help or more information on accreditation checklist requirements and interpretation, contact
the CAP’s LAP Technical Specialists:
• Telephone: 800-323-4040 or 847-832-7000
• Email: [email protected].
The LAP Technical Specialists are medical technologists (most with advanced degrees,
certifications, and management experience) who can coach you in how to ensure compliance,
as well as provide clarity on regulatory requirements. In addition to checklist knowledge, the
LAP Technical Specialists also offer expertise in checklist interpretation and deficiency
response review.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
56
CONDUCTING THE INSPECTION: GENERAL PRINCIPLES AND
MEETINGS
TOPIC
PAGE
Beginning the Inspection
56
General
Principles: How to Inspect 57
Inspection Techniques
58
What to Look at
59
How Much to Look at
60
Inspecting Additional Activities, Disciplines, and Laboratories
61
Using
the Checklists 61
Inspection
of Patient Care Areas
62
Using
the Director Assessment Checklist 62
Meeting with the Laboratory Director
63
Meeting with the Hospital Administrator/Chief Executive Officer (CEO)
63
Meeting with a Representative of the Medical Staff
64
Citing
Deficiencies
65
Deficiencies Corrected
During Inspection
66
Recommendations
67
Completing
the Inspector’s Summation Report (ISR)
68
Pre
-summation Team Meeting
69
The
Summation Conference
70
Concluding
the Inspection
71
Beginning the Inspection
On the day of inspection, the team leader should:
• Plan sufficient time to conduct a thorough inspection. Inspections usually begin at 8 am.
• For unannounced inspections – Contact the laboratory one hour prior to arrival using
the one-hour security notice phone number provided in the inspection packet cover letter.
• For announced inspections – Arrive at the predetermined time.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
57
Upon arrival at the inspection site, the team leader should:
• Present a photo ID to the facility representative (if requested)
• Present the CAP letter identifying the team leader and confirming the date of inspection to
the laboratory director or designee
• Introduce team members and give a brief overview of the day’s schedule
• Request a brief tour of the laboratory.
General Principles: How to Inspect
Inspectors use the checklists and other tools included in the Inspector’s Inspection packet to
conduct the inspection. A listing of the contents of the packet is included in the
Preparing for the
Inspection: Inspection Team section.
The Laboratory-Specific Activity Menu and instrumentation list help the inspector understand the
type and scope of testing within each laboratory section. The inspection checklists are
customized based on the activity menu. Inspectors need to assess whether the listed activities
match the laboratory’s current testing (eg, compare with tests listed in the procedure manuals,
ask about newly introduced tests). If testing is noted which is not included in the activity menu,
inspectors should contact the CAP to obtain additional checklist sections or requirements that
may be needed (refer to the Inspecting Additional Activities, Disciplines, and Laboratories
section below).
Reviewing documents, observing to see if practice matches policy or procedure, and asking
related questions all play an important role in obtaining accurate information about laboratory
practices. Document review may occur in advance of the inspection if the laboratory has elected to
upload select documents to CAP’s Organization Profile. Refer to the Inspection Options
section
and cap.org for additional information.
Inspection team members should:
• Spend more time in the laboratory observing the testing process and asking questions of
bench technologists and supervisors than in a room reading documents
• Continue inspecting while laboratory personnel retrieve needed records
• Rephrase the questions being asked until the request is understood by the laboratory
• Ask open-ended, probing questions that require more than a yes/no answer, such as
“Could you explain how you track QC data?” or “Explain the system you use for…” or “How
do you document…?”. It is more effective than reading the checklist requirement out loud.
CAP inspection team members must avoid the following topics when inspecting a laboratory:
• Financials – discussion of the laboratory’s financial statement
• Billing – discussion of the laboratory’s billing practices
• Proprietary – discussion of the laboratory’s contractual agreements
• Marketing – solicitation of the inspection team’s services available to laboratories (eg,
reference laboratory or consulting laboratory services).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
58
Inspection Techniques
The CAP’s online inspection training course describes a variety of inspection techniques that may
be used when performing an inspection, including:
• “Following a specimen” through the laboratory is an effective technique to address
pre-analytic, analytic, and post-analytic aspects of laboratory testing. This process is
generally followed by review of the laboratory’s documentation. This inspection
technique allows the inspector to not only ensure that the laboratory’s paperwork is in
order, but also to assess personnel knowledge of the laboratory’s processes.
• The “teach me” approach - The inspector selects an analyte or instrument and
laboratory staff “teaches” the inspector about the procedure, process or instrument
operation.
• Read-Observe-Ask-Discover (ROAD) provides a structure for inspectors to conduct an
evaluation of a laboratory’s performance. ROAD icons are placed at the group level
within the inspector’s copy of the checklists. The following icons flag specific instructions
to the inspector:
o Read/review documentation
o Observe procedures/techniques
o Ask probing questions
o Discover the path of a process.
Samples of ROAD instructions:
Read and review policies, procedures, and records that must be looked at during the
inspection.
For example:
• Review the error/accident log; do not simply verify that the laboratory has such a log.
• Review a sampling of the transfusion reaction workups for the past two years.
Observe laboratory practices by watching laboratory personnel in action.
For example:
• Observe a phlebotomy from receipt of requisition to delivery of the specimen to
the laboratory.
• Note if practice deviates from the written policies/procedures.
Ask open-ended, probing questions. This will allow you to:
• Obtain information in their own words, not yours
• Improve your understanding of records and observations
o Assess the laboratory’s interpretation of the
requirements. For example, use questions that begin
with phrases, such as:
o “Show me how …”
o “Tell me about …”
o “What would you do if …?”

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
59
The combination of direct observation and probing questions helps to ensure that:
• Outcomes for any problem areas have been adequately investigated and resolved
(eg, proficiency testing (PT) failures and issues/problems identified through the
quality management process).
• Previously cited deficiencies have been corrected.
Discover additional facts by digging more deeply into one or two areas of special
interest. For example, track a selected specimen from collection to reporting. This will
cover elements in multiple checklist requirements such as:
• The specimen collection manual
• Phlebotomy
• Verbal orders
• Identification of patients and specimens
• Accessioning
• Result reporting, including
o Appropriate reference ranges
o Retention of test records
o Maintaining confidentiality of patient data
o Proper handling of critical results and revisions to reports
What to Look at
Inspectors review relevant documents, such as:
• Procedure manuals
• Quality management system (QMS) documents
• Quality control (QC) records
• Proficiency testing (PT) records
• Instrument maintenance and function check records
• Test method validation and verification studies
• Calibration, calibration verification, and analytical measurement range verification
records
• Method comparison records
• Interim self-inspection records
• Competency assessment
• Delegation of responsibilities.
The Inspector’s Inspection packet contains the following tools to help focus inspection efforts
and identify areas of concerns:
• Previous Inspector’s Summation Report – includes the list of deficiencies from the
previous inspection for the inspector to confirm that all previous deficiencies have been
appropriately addressed to identify recurring deficiencies.
• Proficiency Testing Performance <100% Report (if applicable) - lists by analyte, all PT
scores below 100% during any of the last six testing periods. For each failure,
inspectors should:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
60
o Confirm that the laboratory has conducted an investigation promptly after receiving
the PT report
o Review the testing records to confirm that samples were handled and
reported in the same manner as patient samples
o Review the records for the event following PT failures to confirm that testing
personnel followed the policies and procedures as written. Look for inappropriate
actions such as duplicate testing of PT samples.
o Confirm that the PT results have been reviewed by the laboratory director or
designee in a timely manner.
NOTE: The Proficiency Testing Performance <100% Report is not intended to demonstrate a
laboratory’s enrollment in CAP-accepted PT, as it only includes information on analytes where
proficiency testing performance has been less than 100% in at least one of the previous six
mailings. Analytes that have had 100% performance in the previous six mailings will not be
listed on the report.
How Much to Look at
The review of records, forms and documents is intended to cover a full two-year time frame.
Inspectors of laboratories undergoing an initial CAP inspection should review records from the
time of their application through the inspection date to look for a pattern of compliance with CAP
requirements.
Because there is not sufficient time to look at all records, inspectors must use a sampling
strategy. Inspectors should:
• Consult the Laboratory-Specific Activity Menu and selectively focus on areas of highest
and lowest test volume, common problem areas, and test results with the highest
impact on patient care since the last inspection.
• Refer to the laboratory’s quality management and incident report records to aid in
selection of analytes to review
• Review a representative sampling of analytes or procedures to include:
o Data selected from the beginning, middle, and end of the interval since the last
inspection
o Records in the pre-analytic (order entry and specimen collection, processing and
transport), analytic (procedures, QC, PT, instrument setup, and maintenance),
and post-analytic categories (reports, reference intervals, and critical value
notification); if problems are discovered, review similar records for additional
analytes.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
61
Inspecting Additional Activities, Disciplines, and Laboratories
If the inspection team discovers that testing is being performed under the same CLIA number that is
not listed on the Laboratory-Specific Activity Menu, the inspection team leader or member should:
• Contact the CAP immediately
• Advise the CAP whether a member of the inspection team has the expertise to inspect the
discovered discipline or activities
• Verify that the laboratory is enrolled in appropriate PT for these analytes/activities.
Once notified, the CAP will immediately:
• Determine if inspection of the discovered activities may proceed
• Fax or email a customized checklist to the team member (as needed).
After receiving instructions from the CAP, the inspector should indicate in the Inspector’s
Comments section of Part A of the ISR if the activity/discipline in question has been inspected.
Laboratories that perform testing under a different CLIA number or laboratories that are under
separate administrative and professional direction (eg, blood gas laboratory or pediatric hematology
laboratory) and have not applied in advance for inspection must not be inspected unless
indicated in the inspection packet to do so. The inspector should advise the laboratory director
to submit a formal application to the CAP if indicated. The CAP will schedule an inspection at a later
date. Contact the CAP with any questions regarding the laboratory to be inspected.
Using the Checklists
Each discipline has its own checklist (such as Hematology (HEM)), which is used along with
the All Common (COM) to inspect each laboratory section.
One copy of the Laboratory General Checklist (GEN) is provided to each inspection team. While
this checklist may be assigned to one inspector, each inspector should verify compliance with the
safety and physical facility requirements for their designated areas and report findings to the
assigned GEN inspector.
Inspectors should focus on groups of requirements using the ROAD instructions included within
the checklist and other inspection techniques mentioned above instead of going through the
checklist requirement by requirement.
The Evidence of Compliance (EOC) section of a checklist requirement lists suggested ways to
show compliance; some elements are required. The word “AND” in the list indicates that more
than one element may be needed to demonstrate compliance. For example, the EOC for
COM.01500, Alternate Performance Assessment reads:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
62
• List of tests defined by the laboratory as requiring alternative performance assessments
AND
• Records of these assessments.
If the intent of a checklist requirement is not clear, inspectors may contact the CAP for
clarification prior to or during the inspection by email at [email protected]
4040 ext. 6065 or 847-832-7000, during the hours between 8:00 AM to 5:00 PM CST.
Inspection of Patient Care Areas
During the course of the inspection, some team members will visit patient care areas when
appropriate based on current CAP or facility safety measures. Examples include:
• Observation of transfusion of blood components issued by the laboratory
• Point-of-care testing (if under the laboratory’s CAP/CLIA number)
• Observation of phlebotomy blood draws performed by laboratory staff
• Observation of arterial blood gas specimen collection and testing.
Meeting with direct health care providers and observing the tests and procedures they perform
can help inspectors determine compliance with checklist requirements and evaluate oversight of
those services.
The visit should include:
• Review of laboratory records within the patient medical record
• Assessment, through interviews, of laboratory responsiveness to clinical needs
• Identification of concerns to be communicated to the laboratory director.
Using the Director Assessment Checklist
The team leader or team member who is qualified and trained to be a team leader must
complete the Director Assessment Checklist (DRA). This checklist:
• Evaluates the qualifications of the laboratory director and the effectiveness of the
laboratory director in implementing the Standards for Laboratory Accreditation
including requirements to evaluate the overall performance characteristics of the
laboratory. Assists the team leader to recognize and document systemic
problems with the laboratory’s QMS.
• Contains instructions for conducting interviews with the laboratory director, hospital
administrator/chief executive officer (CEO), and representative of the medical staff.
• Focuses upon those aspects of the laboratory that are at the core of quality: the
laboratory director’s responsibilities, the QMS, and the laboratory’s relations with the
institutional medical staff and administration.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
63
The interviews conducted by the team leader with the laboratory director, hospital
administrator/CEO, and representative of the medical staff provide some of the
information needed to complete the inspection with the DRA Checklist. The interviews
may be conducted in-person or virtually. They are an essential part of the inspection. If,
for any reason, an interview cannot be conducted, the team leader should report the
circumstances in the Inspector’s Summation Report (ISR).
The team leader may record information from these interviews in Part A of the ISR.
Deficiencies, if found, are to be cited on the DRA Deficiency page of the ISR, Part B.
Meeting with the Laboratory Director
Meeting with the laboratory director helps determine if the laboratory director has sufficient
responsibility, authority, and involvement in the operations of the laboratory. The inspector
should allow at least 15 to 20 minutes for the meeting. If the laboratory director is not present for
the inspection, the inspector should try to conduct this interview by telephone or other means.
Conversations with technical staff, administration, and the representative of the medical staff
may be used to validate the laboratory director’s involvement.
The interview is an opportunity to:
• Evaluate the laboratory director’s activities as listed in the DRA Checklist and the
Standards for Laboratory Accreditation
• Review any problems that the inspection might serve to resolve (eg, workspace
issues, staffing shortages)
• Determine if the laboratory director also functions as a technical supervisor,
clinical consultant, general supervisor, or as one of the testing personnel.
Meeting with the Hospital Administrator/Chief Executive Officer (CEO)
Meeting with the hospital administrator/chief executive officer (CEO) provides an opportunity to
extend the CAP’s appreciation for the facility to participate in the accreditation program and to
record an evaluation of the laboratory from the administration’s viewpoint.
The team leader should allow approximately 15 to 20-minute discussion and should understand
the laboratory’s operations beforehand.
For hospital laboratory inspections, the team leader may find it useful to meet with the
institutional quality assurance manager (sometimes called the quality/risk manager). This
individual may have insights into the laboratory-related, patient care issues.
For independent laboratories, the team leader should meet with an executive from the parent
organization.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
64
The interview is an opportunity to:
• Ascertain the administration’s perception of the laboratory service (eg, if the
laboratory service level is appropriate to the requirements for the institution)
• Discuss administration’s view of the laboratory director’s role in ensuring high-
quality laboratory services to fulfill the needs of the institution’s patients and
clinicians
• Determine if the institution gives the laboratory director the authority to
fulfill the laboratory director’s responsibilities under the CAP and CLIA
• Inquire to what extent the pathologists participate in hospital-wide committees
• Determine how effective pathologists are in working with the medical and
administrative staffs
• Identify areas of conflict or challenges confronting the laboratory that are
known to administration.
Discussion points during the interview should include:
• The goals of the CAP’s accreditation programs: education, laboratory improvement, and
the establishment of best practices in laboratory medicine based on input from national
experts
• The role of PT in the program
• The responsibility of the laboratory director for the overall operation of the laboratory, per
the requirements of the CAP’s accreditation programs and CLIA regulations
• Express appreciation that the organization has chosen the CAP as its
laboratory’s accreditation provider.
The interview should include a discussion of all laboratories being inspected (eg, point of care,
special function and satellite laboratories). The CAP prohibits discussion of the laboratory’s
financial and/or contractual arrangements.
For facilities participating in the Biorepository Accreditation Program, the team leader may
choose to interview a member of administration if available, but this step is not required.
Meeting with a Representative of the Medical Staff
Meeting with a representative of the medical staff can provide an opportunity to determine
whether the laboratory director and laboratory staff have an established working relationship
with the medical staff and are effectively supporting patient care. For laboratories associated
with organized medical staffs, it is important for the team leader to interview the chief of the
medical staff (or other knowledgeable medical staff representative, such as the chief medical
officer or a physician who uses the laboratory’s services frequently). The team leader should
allow for a 15 to 20-minute discussion and should understand the laboratory’s operations
beforehand.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
65
The interview is an opportunity to:
• Evaluate the effectiveness of the scope, quality, and timelines of the laboratory
services to meet the patient care needs of the hospital
• Assess the contribution of the pathologists and laboratory staff to teaching conferences
and meetings
• Determine how well the medical staff and pathologists work together to resolve problems
• Judge the medical staff perception of the effectiveness of the laboratory director and
other pathologists
• Determine if the laboratory director has sufficient authority to fulfill the needs of the
medical staff and their patients.
Citing Deficiencies
Inspectors must cite deficiencies when the intent of a checklist requirement is not being met.
Inspectors should not expect the laboratory to do things exactly as they are performed in the
inspector’s facility. However, when the laboratory’s processes or procedures are not in
compliance, deficiencies must be cited.
Examples include:
• When a required policy or procedure does not exist
• When the written policy or procedure is not being followed
• When results or corrective actions are not recorded
• When a required record of review does not exist
• When the procedure is ineffective or inappropriate laboratory practice is in place
• When the records are incomplete or missing
• For any non-compliance issues related to personnel qualifications, proficiency testing,
QC/QA, and director oversight.
When records are incomplete, inspectors should:
• Determine whether the degree of partial compliance is likely to have adverse effects on
test accuracy, patient care, or worker safety
• Determine if laboratory staff was aware of the inconsistency
• Look for evidence of corrective actions.
Deficiencies are also cited when systemic problems exist. For example, when a pattern of
missing temperatures on the weekend without corrective actions is evident, then a deficiency
must be cited. When systemic or serious issues are identified, the inspection team members
must bring them to the attention of the team leader, who will determine if a deficiency needs to
be cited from the DRA Checklist for the related laboratory director responsibility.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
66
Examples of serious or systemic issues include:
• QMS not implemented across the laboratory
• Inconsistent quality control and corrective action
• Improper handling of proficiency testing materials or lack of follow-up for unacceptable
results
• Lack of validation or verification records for new tests or instruments
• Unsafe practices compromising the safety of personnel
• Duties delegated by the laboratory director not being effectively performed
• Write the checklist item number and checklist requirement phase, followed by a brief
description of the reason for the deficiency
• Provide specific details about the nature of the non-compliance with stated examples
(eg, dates involved, analytes affected, instruments or kits used, name of record or
probation, etc.), whenever possible and not just restate the checklist requirement as
written
• Write/print legibly.
When the inspector and the laboratory representative interpret a checklist item differently, they
are encouraged to call the CAP’s technical support line at 800-323-4040 or 847-832-7000
during the inspection.
A three-way dialogue between the inspector, laboratory, and accreditation program technical
specialist often helps clarify the intent of the checklist item. Contacting the CAP can result in fewer
improperly cited deficiencies and laboratory deficiency challenges post-inspection.
Deficiencies Corrected During Inspection
Some deficiencies may be corrected while the inspectors are still on site. Correction on site is
a relatively rare occurrence and includes minor corrections, such as signing one or two
procedures, inserting minimal changes in a procedure, or writing a policy to match existing
practice. In all cases, inspectors must cite the deficiency and indicate on the Part B
Deficiency form (ISR pink sheet) how the deficiency was corrected.
Other more extensive deficiencies cannot be corrected during inspection. Examples include:
• Lack of a Quality Management System
• Lapse in performance or review of QC or proficiency testing, or implementation of a new
or significantly changed procedure
• A change to a process, policy, or procedure that requires additional training or retraining
of personnel
• When previous patient results must be evaluated for impact to patient care (eg, when
expired reagents are found to be in use or when incorrect result calculations are
identified)
• Recurring deficiencies.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
67
Deficiencies corrected during inspection are deficiencies and will remain in the
laboratory record. The CAP may request documentation from the laboratory concerning
how a deficiency was corrected during inspection; for Phase II deficiencies, both a
corrective action plan and evidence to support implementation may be requested.
Recommendations
Recommendations are considered suggestions for laboratory improvement and are listed on the
“Recommendations” yellow page of the ISR. Recommendations may be given in the following
situations:
• When a laboratory is in compliance, but can improve its process
• When an inspector has a suggestion that does not pertain to a specific checklist
requirement.
Recommendations may not substitute for deficiency citations if the laboratory is not in
compliance. Laboratories are not required to take corrective action in response to
recommendations, unless requested to do so later by the CAP. Based on the information
provided with the laboratory’s response, recommendations may be converted to deficiencies.
The following are examples of recommendations:
• “List the acceptable ranges of temperature dependent equipment on recording logs in
addition to the maintenance procedure to allow staff to easily identify variances”
• “Store personnel competency records in a centralized location rather than have portions
of the records in multiple sections”
• “Standardize document control processes across all sections of the laboratory.”
Recommendations are recorded on the designated yellow pages of the Part B section of the
ISR. Inspectors should:
• Write the checklist number and checklist requirement phase (if applicable),
followed by the recommendation
• Write or print legibly
• Discuss the recommendation with the laboratory personnel prior to the Summation
Conference.
Recommendations need not be presented at the Summation Conference. In situations where there
are a large number of deficiencies, it is recommended that the team focus on the deficiencies and
not present the recommendations because this can lead to confusion and cause the summation
conference to be unduly long.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
68
Completing the Inspector’s Summation Report (ISR)
The Inspector’s Summation Report (ISR) is used to record the findings of a CAP inspection. It
consists of two parts.
• Part A- General Summary- used to report any fundamental disparities between the
intent of the Standards and the activities of the laboratory and of the role of the
laboratory director. The inspector’s confidential comments, listed in Part A, are pivotal in
accreditation decisions, particularly those relating to denial of accreditation. This
narrative section is completed by the Team Leader with input from all team members.
The Team Leader provides explanatory comments in the ISR Part A regarding
unexpected testing encountered, as well as inappropriate checklists included in the
packet.
• Part B – Deficiency Summary- includes the Signature Page, Deficiency forms (pink
pages), and Recommendation forms (yellow pages). The laboratory director and team
leader sign the Signature Page at the conclusion of the inspection to attest that the
laboratory received a copy of the ISR. Each Deficiency form includes space to record
detailed comments to support the cited deficiencies and an attestation statement to be
signed by the inspector.
Tips for completing the ISR:
• The Team Leader provides explanatory comments in the ISR Part A regarding
unexpected testing encountered, as well as inappropriate checklists included in the
packet.
• Team member inspectors should only use the assigned ISR pages for each discipline. It
is not appropriate to cross out sections or include additional checklist titles on the ISR
pages. An extra (blank) pink page may be found at the end of the ISR packet and
copied as needed to record additional deficiencies.
• Each inspector must complete the bottom of the deficiency form attesting to the
completeness of the inspection, the confidentiality of information, and the lack of a
conflict of interest.
• If multiple inspectors participated in the inspection for the same discipline/checklist,
additional members are to be listed on the reverse side of the form.
• If corrections need to be made to remove a deficiency (eg, appropriate records have
been provided to show that the laboratory was in compliance), the deficiency should be
redacted from the pink page using a single strikethrough line, initialed and dated by the
inspector.
• If the deficiency was corrected during inspection, the inspector should write “corrected
during inspection” next to the deficiency and describe how the laboratory corrected the
deficiency.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
69
NOTE: If serious deficiencies or systemic issues are identified or any question from Part
A is answered “NO”, the Team Leader must ensure that the appropriate Laboratory
General or section-specific checklist requirements relating to the issue are cited, as well
as the DRA Checklist requirement(s) related to laboratory director responsibility.
Pre-summation Team Meeting
The pre-summation team meeting begins with the pre-summation preparation, a 30 to 60-
minute private meeting between the team leader and the inspection team members. The goals
of the meeting are to ensure that the written inspection reports are complete, that the reports
are consistent across the team, and that their oral summaries will reflect the written report.
During the meeting, the team leader should:
• Resolve team member questions
• Ensure consistency in recording similar findings (eg, deficiency versus recommendation)
• Identify serious deficiencies that may jeopardize patient care and systemic problems
where inspectors cited the same or related deficiencies in multiple laboratory sections.
Before concluding the pre-summation meeting, the Team Leader should ensure that:
• All areas of the laboratory have been inspected
• Every inspection team member has completed (pink) deficiency and (yellow)
recommendation forms that correspond to the laboratory section(s) for which he/she is
responsible and have provided contact information on the back of the appropriate forms
• Each pink and yellow copy has been signed and dated by the respective inspection
team member attesting that the inspector has no conflict of interest and that all relevant
checklist requirements have been evaluated.
• Appropriate checklist items have been cited and the correct deficiency numbers are
listed on the pink deficiency sheets
• Any changes that may have occurred during the pre-summation conference (additions
or deletions in deficiencies) are communicated to the appropriate laboratory
representatives
• The “This laboratory section has no deficiencies” box and/or “No recommendations
for this section” box have been checked as applicable
• No Part B deficiency (pink) or recommendation (yellow) form is missing or has been left
blank or unsigned.
• All deficiency and recommendation pages have been accounted for by comparing the
completed pages to the list that appears on the pink inspector Summation Report (ISR)
Page Index. If the inspector is concerned that a provided checklist is not needed, the
CAP must be contacted prior to marking any ISR page “not used.”

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
70
The Summation Conference
The summation conference may be the most important part of the inspection. It is the final
opportunity for interaction between the inspection team, the laboratory staff, and administration.
The summation conference in coordination with the laboratory should be scheduled for a time
when personnel involved in the inspection can attend, such as the end of the workday. The
inspection team should identify areas that require improvement, share information regarding
how other laboratories accomplish compliance, and make recommendations for changes to
patient care services.
Attendees should include the:
• Laboratory director
• Key laboratory personnel
• Hospital administrator
• Chief of the medical staff/medical staff representative, if applicable.
At the start of the summation, the team leader should state the objective of the CAP’s laboratory
accreditation programs. Talking points include:
• The College of American Pathologist Laboratory Accreditation Program seeks to
improve laboratory medicine for the benefit of patients through voluntary, educational,
peer review
• Regulatory requirements must be met, but these are not the only goals of the program
• The primary objective is not to find deficiencies, but to assist the laboratory in validating
its ongoing process and assessing their compliance with CLIA and CAP checklist
requirements.
The laboratory should encounter no surprises when the inspection report is presented. To
ensure this, it is critical for inspectors to have discussed their findings with the supervisors
during the inspection and/or at the conclusion of each section.
Each team member should:
• Begin with a brief self-introduction and word of thanks for the staff that assisted them in
the inspection process
• Present the inspection findings in a brief and professional manner, including the
deficiencies identified and areas where the laboratory did particularly well
• Allow time to answer questions from the laboratory team.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
71
The summation conference is also an appropriate time to:
• Report any unresolved differences regarding the interpretation of the Standards or
checklist requirements. Unresolved differences should be noted by the Team Leader in
Part A of the ISR
• Recognize any positive aspects of the laboratory encountered during the inspection.
Talking points for the team leader at the close of the summation conference:
• Approximate the total number of checklist requirements that were used to inspect the
laboratory so that those in attendance can put the number of identified deficiencies into
perspective.
• Explain that deficiency responses, documentation of corrective action, and
documentation of the laboratory director’s signatory approval of the responses are to be
submitted to the CAP within 30 calendar days of the inspection date. An
accreditation decision usually takes approximately 75 days after the inspection.
o Phase I deficiencies require a written response.
o Phase II deficiencies require a response, a plan of corrective action, and
supporting documentation that demonstrates implementation.
o Deficiencies corrected during inspection do not require a response but are
counted as deficiencies.
• Express the team’s gratitude and extend congratulations to the laboratory and the staff
for participation in the program and their work in preparing for and participating in the
inspection. Acknowledge the hospitality and cooperation of the staff during the process.
• Thank the laboratory director for supporting the CAP accreditation program.
• Explain that the copy of the handwritten Inspector’s Summation Report Form left at the
end of the inspection (not including Part A comments) is the official report from the CAP.
There will be no printed list of deficiencies sent from the CAP to initiate the laboratory’s
corrective action and response to the CAP.
It is not necessary to present DRA deficiencies at the Summation Conference if they were
previously discussed with the laboratory director.
Concluding the Inspection
The team leader has several additional responsibilities immediately after the summation:
• The laboratory director and the inspection team leader must both sign page 3 of
the ISR-Part B-Deficiency Signature Page.
• Arrange for the inspection checklists, other documents that were used during the
inspection, and any remaining inspection materials to be discarded confidentially (eg,
immediately shredded). All materials except the list of previous deficiencies may be left
with the laboratory to shred. The list of previous deficiencies contains the Part A

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
72
confidential comments from the previous inspection team leader and therefore should
not be left at the laboratory.
• Ensure that the Inspector Comments section of Part A of the ISR includes:
o The team leader’s opinion of the quality of the laboratory and ability of the
laboratory to maintain continuous compliance
o Issues of disagreement between the inspector(s) and the laboratory staff
o Anything else that may impact the accreditation decision.
• Ensure that each page of the ISR Part B has been photocopied and left with the
laboratory director (do not leave Part A comments)
• Provide the envelope that contains the deficiency response forms and instructions to the
laboratory director or designee.
If the inspection team discovers that they forgot to cite a deficiency after the inspection, the
team leader must notify the CAP immediately upon this discovery and provide details of the
deficiency in writing.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
73
AFTER THE INSPECTION: INSPECTION TEAM
TOPIC
PAGE
Process
for Returning the Inspector’s Summation Report 73
Claim
for Inspection Reimbursement 74
Team Leader and Team Member Evaluation Forms
74
Remaining Inspection Materials
74
Inspector Feedback Process
74
Process for Returning the Inspector’s Summation Report
All deficiency (pink) and recommendations (yellow) Inspector’s Summation Report (ISR) pages
(except for the “extra” page), along with pages 1-3 of the ISR Part A and the ISR Index Page
must be returned in the mailing envelope provided. The mailer has a United Parcel Service
(UPS) prepaid label and can be used in the 48 contiguous states.
• For laboratories located in the US, except Hawaii and Alaska, the ISR must be returned
within two business days after the inspection.
• For all other inspections, the ISR must be returned within two days after returning to
the contiguous 48 states.
• For non-routine inspections and any inspection that has identified significant,
concerning issues, the ISR must be returned within 24 hours.
Returns can be:
• Sent from the team leader’s facility mail center for pick-up by UPS
• Given to any UPS driver making a regular pickup
• Taken to any UPS authorized shipping location.
To locate the nearest UPS location or to arrange for a special pickup, either access the UPS
website (www.ups.com) or call 1-800-PICK-UPS (800-742-5877).
For shipping internationally, the following options should be considered:
• Searching the ups.com website
• Calling 1-800-782-7892
• C
ontacting an alternative local carrier that ships to the US.
The Claim for Inspection Reimbursement and the Team Leader/Member Evaluation forms may
be returned to the CAP with the ISR or within 90 days.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
74
Claim for Inspection Reimbursement
Return of the completed ISR must not be delayed while waiting for the collection of expense
claims because this can delay the accreditation process for the inspected laboratory.
The Claim for Inspection Reimbursement form includes instructions for expenses that are
reimbursed, maximum allowable expenses, and receipt requirements. Reimbursement claims
should be submitted within 90 days of the inspection.
Team Leader and Team Member Evaluation Forms
Critique of the inspection process and experience by both team leaders and team members is
essential feedback for the improvement of the accreditation programs. Team leaders should
complete the Team Leader Evaluation questionnaire and each member of the inspection team
should complete a Team Member Evaluation questionnaire.
Remaining Inspection Materials
Any remaining inspection packet materials, including checklists, must be discarded. Laboratory-
specific information must be shredded to maintain confidentiality.
Inspector Feedback Process
To improve the quality of inspections, the CAP provides inspection team leaders with feedback
on the performance of their inspection team once the inspected laboratory has completed the
post-inspection review process and the accreditation decision has been made. This feedback
includes, as applicable:
• Comments from the inspected laboratory
• Information on trends noted based on expungement of any deficiencies
• Information on conversion of recommendations to deficiencies
• Other comments identified during the post-inspection review of the inspection findings
and corresponding laboratory responses.
Feedback may be provided to the team leader via letter, mail, or phone call. The team leader
should share the information with his/her team.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
75
AFTER THE INSPECTION: LABORATORY
TOPIC
PAGE
Responding
to Deficiencies 75
Challenging
a Deficiency 77
Deficiencies
Corrected During the Inspection 77
Deficiency
Response Review 77
Immediate
Review Criteria 78
Accreditation
78
Post
-inspection Critique 79
Probation
Categories 80
Denial
or Revocation of Accreditation 81
Appeals
81
Responding to Deficiencies
Before the inspection, the laboratory will receive a Laboratory Inspection Packet that contains
instructions for online submission of responses to any deficiencies cited during the inspection. In
the event an online submission is not an option, contact the CAP headquarters at 800-323-
4040.
At the conclusion of the inspection, the inspection team will give the laboratory:
• A blue envelope containing instructions for responding online to deficiencies cited
• A copy of the Inspector’s Summation Report (ISR) with the deficiencies and
recommendations listed.
The deficiencies will also be available online approximately one week after the
inspection after the ISR is processed by the CAP. Laboratories can print a typed replica of
the inspection findings by logging into cap.org
in e-Lab Solutions Suite under Accreditation-
Accreditation Reports-Inspection Summary Report. The laboratory must submit
appropriate responses to the CAP within 30 calendar days of the inspection. Failure to
respond may result in denial or revocation of accreditation.
Phase I deficiencies require a response that describes the corrective action taken. Supporting
documentation of deficiency correction is not required unless specifically requested by the CAP.
Phase II deficiencies require a response and supporting documentation to demonstrate that the
laboratory is now in compliance. Responses should explain the purpose of each document

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
76
submitted. Deficiencies noted as “corrected during inspection” require no response unless
specifically requested by the CAP.
Examples of supporting documents for Phase II deficiencies:
• New or revised policies or procedures with evidence of the laboratory director’s review
and approval (with the portions pertained to the deficiency underlined or otherwise
indicated)
• Quality control, calibration, maintenance records, and instrument printouts
• Log sheets with recorded data (blank log forms are unacceptable)
• Purchase orders, work orders, photos, diagrams, and floor plans
• Evidence of staff review or retraining on new, revised, or existing procedures.
Recommendations are suggestions for improvement, and the laboratory is not obligated to
implement or respond to them. NOTE: A recommendation that should have been cited as a
deficiency during the inspection will be changed to a deficiency after the inspection, and
the laboratory will be required to respond. Recommendations that have been converted
to deficiencies will be listed on the Accreditation Letter that is sent to the laboratory by
the Regional Commissioner.
Protected health information (PHI) must be redacted from submitted documents in accordance
with HIPAA requirements. The following patient data must be de-identified prior to submission:
• Name
• Address
• Any elements of dates, excluding the year, for dates directly related to an individual,
including birth date, admission date, discharge date, date of death
• Telephone numbers
• Fax numbers
• Email addresses
• Social Security number
• Medical record numbers
• Health plan beneficiary numbers
• Account numbers
• Biometric identifiers, including finger and voiceprints
• Device identifiers and serial numbers
• Certificate or license numbers
• Vehicle identifiers and serial numbers, including license plate numbers
• Web Universal Resource Locators (URLs)
• Internet protocol (IP) addresses
• Full-face photographs or comparable images
• Any other unique identifying number, characteristic, or code.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
77
Challenging a Deficiency
The laboratory may challenge any deficiency cited by the inspection team. The intention to
challenge a deficiency must be clearly stated within the response. This can be accomplished
by:
• Indicating the challenge within the submitted online deficiency response
• Providing an explanation for the challenge within the response
• Attaching documentation to support the claim that the laboratory was in compliance at
the time of the inspection. This can be accomplished by uploading a file in your online
deficiency response form.
Supporting documentation is required for challenges to both Phase I and Phase II deficiencies.
Challenges must be made at the time initial responses are submitted. Submission of
records modified or created on the date of or after the inspection are not adequate to support a
challenge to a deficiency.
Acceptance of a challenge and subsequent deficiency removal is at the discretion of the
reviewing commissioner. If the challenge is not accepted, additional documentation showing
correction of the deficiency may be required, and the deficiency will appear in the listing of
deficiencies routinely included in the accreditation packet. Deficiencies that have been
approved for removal by the reviewing commissioner will not appear on the final list of
deficiencies and are not part of the permanent inspection record. Challenges to deficiencies
will not be accepted after the accreditation decision has been made.
Deficiencies Corrected During the Inspection
Deficiencies corrected during inspection will remain in the record as deficiencies. The
CAP reserves the right to request documentation from the laboratory concerning how a
deficiency was corrected during inspection; for Phase II deficiencies, both a corrective action
plan and evidence to support implementation may be requested.
Deficiency Response Review
After the inspection, the laboratory director must ensure that:
• Responses for each deficiency are submitted online to the CAP within 30 days of the
inspection date
• Any resolved differences and challenges to the deficiencies cited are addressed in the
laboratory’s deficiency response. This includes supporting documentation to
demonstrate that the laboratory was in full compliance at the time of the inspection.
• The Laboratory Director Signature Page is signed
• A copy of all deficiency responses is kept on file.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
78
The CAP performs the remaining steps of the accreditation process:
• Using the information provided by the inspector, a technical specialist evaluates the
deficiency responses for appropriateness and completeness. If additional information is
needed to evaluate compliance, a request for documentation is sent to the laboratory
via email. The laboratory is expected to respond online within 10 days.
The assigned CAP reviewing commissioner will also review the responses. The reviewing
commissioner:
• Evaluates the acceptability of each response
• May request additional information from the laboratory prior to making an accreditation
decision
• Determines whether challenged deficiencies will be removed
• May change a recommendation to a deficiency (if warranted)
• May add a deficiency based on comments that were included in the Part A Summary if
the laboratory was clearly not compliant at the time of the inspection
• Makes an accreditation decision recommendation to the Accreditation Committee
• Notifies the laboratory that accreditation is recommended.
Immediate Review Criteria
The CAP’s accreditation programs have established criteria for expedited processing by the
CAP staff and the regional commissioner. Immediate review occurs when a laboratory is cited
for deficiencies on more than 2.5% of the total applicable Phase II requirements or when a
directorship issue is cited.
It is often difficult for laboratories with large numbers of deficiencies to correct them within 30
days. The reviewing and/or regional commissioners may:
• Communicate with the laboratory director and the state commissioner to determine if
correction is possible
• Recommend to the Accreditation Committee a focused re-inspection of the problem areas
• Recommend probation, suspension, or denial of accreditation.
Accreditation
The Accreditation Committee grants accreditation when the laboratory has provided acceptable
responses to Phase I and Phase II deficiencies and satisfactorily documented correction of all

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
79
Phase II deficiencies. Laboratories granted accreditation may be required to meet additional
requirements to maintain accreditation, such as:
• Submitting records at defined intervals supporting ongoing correction of deficiencies
• Undergoing a successful nonroutine inspection within a specified time-period to confirm
ongoing compliance
• Undergoing a successful routine on-site inspection after a virtual inspection within a
specified time-period to complete the full accreditation cycle.
For laboratories with too many deficiencies to be corrected within a reasonable period, the
Accreditation Committee may place the laboratory on probation or decide to deny or revoke
accreditation. Refer to the Probation Categories
section below for more information.
Once the Accreditation Committee makes an accreditation decision, the CAP will mail an
accreditation packet to the laboratory. The accreditation packet includes:
• The accreditation certificate and accreditation letter to the laboratory director (with
copies of the letter to the administration where applicable)
• Letter of accreditation that specifies the CAP-accredited disciplines/sub-disciplines,
CMS specialties/subspecialties, and requirements for continued accreditation
• Final list of deficiencies
• Press release (including instructions on how to use the CAP accreditation mark).
Accreditation is initially valid for two years from the date of the first inspection and is
renewable every two years on the accreditation anniversary date. Should accreditation
processing go beyond the accreditation’s anniversary date, the state of the laboratory’s
accreditation remains unchanged until that decision is made. During this period, if a
laboratory receives requests from an outside entity to demonstrate continuing accreditation, a
letter may be obtained from the CAP to verify its accreditation status.
Laboratories should retain the final list of deficiencies on record for review by other
accrediting agencies (eg, The Joint Commission). A copy of the list of deficiencies is provided
to the next inspection team to confirm continued compliance.
Post-inspection Critique
After the inspection, the CAP sends the laboratory director a Post-inspection Critique
questionnaire. This questionnaire:
• S
erves as an ongoing quality assurance tool for the inspection process
• Is used by the CAP to make continuous improvements at every level.
The laboratory director is strongly encouraged to solicit feedback from laboratory personnel who
participated in the inspection, and to return the questionnaire to the CAP within three months of
the inspection.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
80
Probation Categories
The Accreditation Committee may place a laboratory on probation or any section of a laboratory
on suspension. During probation, a cited laboratory or section is allowed to provide testing as an
accredited laboratory. A suspended section is not allowed to provide accredited testing. When a
probation or probation with suspension decision is made, agencies that recognize CAP
accreditation, including but not limited to the Centers for Medicare and Medicaid Services
(CMS) and The Joint Commission, are notified. The laboratory will remain on probation until the
Accreditation Committee removes the probationary status.
Probation may occur for conditions that do not appear to pose a substantial risk of harm to
patients or to laboratory personnel; for instance:
• Available facts are insufficient to determine compliance
• The Accreditation Committee wishes to monitor the progress of deficiency correction
• Laboratory conduct is contrary to the policies of the CAP
• The Accreditation Committee has denied or suspended the accreditation of specific
sections of a laboratory.
Probation with Immediate Jeopardy may occur for conditions that demonstrate potential
serious adverse effects on safety to the public and/or laboratory staff and immediate action is
warranted, such as:
• Lack of laboratory director oversight
• Patient/specimen identification issues
• QC issues that place patients at risk
• International normalized ratio (INR) issues.
Laboratories placed on probation with immediate jeopardy are given five business days
to satisfactorily correct the deficiencies. The Accreditation Committee will review the
laboratory’s response and reconsider the accreditation status. This may result in
revocation.
Probation with Suspension may occur if either of the following conditions is present:
• The laboratory has deficiencies that pose a substantial risk of harm to patients or to
laboratory personnel, and the Accreditation Committee needs time to evaluate the
situation further or concludes that the deficiencies can be corrected within a specified
period
• The laboratory has failed to enroll in an approved PT program
• The laboratory has failed to meet PT performance criteria.
In general, the suspension will be resolved within 45 days. The Accreditation Committee will
decide to either:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
81
• Reverse the suspension of the specific section based on the laboratory’s sufficiently
addressing the issue cited OR
• Revoke the accreditation of the entire laboratory. The laboratory using its CAP
accreditation to meet regulatory standards must officially cease all testing in all sections
affected.
Denial or Revocation of Accreditation
Accreditation is denied or revoked when the laboratory fails to meet any of the standards within
the CAP’s accreditation programs or any other requirement for continued participation in the
accreditation programs, and it cannot institute corrective action in the time allowed. The
checklists represent the requirements for meeting the Standards. Failure to correct cited
deficiencies can be the basis for determining that a laboratory does not meet the intent of one or
more of the Standards.
Laboratories undergoing formal denial or revocation of CAP accreditation will receive notification
by mail for next day delivery. Agencies applicable to the laboratory accepting CAP accreditation,
including but not limited to the CMS or the Joint Commission, will be notified.
A laboratory that has had accreditation denied or revoked may reapply for accreditation six
months following the date of notification of denial or revocation.
Appeals
The laboratory may appeal denial or revocation within 30 days of receiving written notice of that
decision. Appeals must be accompanied by appropriate documentation. A request for
reconsideration shall not stay the denial of accreditation. Request for information regarding
appeal procedures must be directed to the Senior Director, Accreditation and Regulatory Affairs
at the CAP headquarters at 800-323-4040 ext. 7243 or 847-832-7243.
F
or additional detailed information concerning accreditation, probation, suspension, denial,
revocation, and appeals, refer to the CAP Accreditation Programs Policies Manual on cap.org
in e-LAB Solutions Suite under Accreditation Resources – Accreditation Manuals/Retention
Guidelines.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
82
MAINTAINING ACCREDITATION
TOPIC
PAGE
Terms
of Accreditation Form 82
Maintaining
CAP Accreditation Information 84
Proficiency
Testing Participation 84
Self
-inspection 84
Anniversary
of Accreditation 84
CAP
Reporting to Organizations and other Government Agencies 85
CAP
Website Resources 86
e-LAB Solutions Suite 86
Performance Analytics Dashboard 86
e-Alerts 86
My Profile 87
LAP Policies 87
Terms of Accreditation Form
As a condition of CAP accreditation, the laboratory director must sign the Terms of Accreditation
form attesting that the laboratory will comply with the conditions listed.
A CAP-accredited laboratory must:
• Provide a trained inspection team comparable in size and scope to that required for its
own inspection at least once during the two-year accreditation period, if requested by
the regional and/or state commissioner.
• Participate annually in a CAP-accepted proficiency testing (PT) program, as applicable.
• Promptly notify the CAP:
o Of any changes in directorship, location, ownership, name, insolvency, or
bankruptcy in the 30 days prior to the change. Laboratories subject to the US CLIA
regulations must also notify the Centers for Medicare and Medicaid Services (CMS)
of pertinent changes.
o Of any changes in the laboratory's activity menu prior to beginning that testing or
implementing scope of service/analytic method changes, or the laboratory
permanently or temporarily discontinues some or all testing,
o If the laboratory discovers laboratory personnel actions that appear to violate
national, federal, state (or provincial), or local laws that regulate laboratories.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
83
• Have a written procedure for employees to communicate concerns about quality and
safety to management, and for management to investigate employee complaints.
Incorporate corrective or preventive actions into the laboratory quality management
program.
• Cooperate in any CAP investigation or inspection, and promptly notify the CAP if the
laboratory becomes the subject of:
o An investigation by a government entity (including national, federal, state (or
provincial), local, or foreign) or by another accreditation organization.
o A validation inspection
o Adverse media attention related to laboratory performance.
• Authorize the CAP to release its inspection and PT data and other information required
by law to the appropriate regulatory or oversight agencies (eg, the CMS, The Joint
Commission).
• If the laboratory is subject to the US CLIA regulations:
o Make available on a reasonable basis the laboratory's annual PT results
upon request of any person
o Allow the CMS or its agent to perform a validation or complaint inspection at any
time during the laboratory's hours of operation and permit the CMS to monitor
the correction of any deficiencies found through such an inspection
o Obtain a CLIA Certificate of Accreditation and pay all applicable fees as a
CLIA-certified laboratory if it will use CAP accreditation to meet CLIA
certification requirements.
• Submit a completed Self-Inspection Verification Form in the interim year.
• Submit only documentation and other materials to the CAP that have been de-
identified of all protected health information (PHI) in accordance with the requirements
of the Health Insurance Portability and Accountability Act of 1996 and its
implementing regulations unless the laboratory must submit PHI to the CAP to respond
to a deficiency or patient complaint.
• Accept and adhere to the Certification Mark Terms of Use/Agreement for CAP
Accredited Mark and Design if the laboratory is, or will use the CAP Certification Mark of
accreditation. The agreement may be downloaded from cap.org
.
• Refrain from copying or distributing the CAP Checklists or any content thereof
except for use by inspectors in conducting a CAP inspection and by the laboratory in
preparing for such an inspection.
• Laboratories participating in the Laboratory Accreditation Program are required to pay for
CAP annual accreditation fees based on the applicable disciplines/sub-disciplines of the
lab. Those fees are set based on complexity points, test volume points, base fee and
specialty fees that apply at the time of the billing month for the site. Find further
information about specific fees by emailing [email protected]
.
• Laboratories participating in any CAP Specialty Programs, including Reproductive
Laboratory Accreditation, Forensic Drug Testing Accreditation, Biorepository
Accreditation, System Inspection Option, are required to pay for these annual
accreditation fees that apply at the time of the billing month for the site.
• International laboratories are subject to pay business class airfare for any United States-
based inspector that inspects on-site.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
84
Maintaining CAP Accreditation Information
As indicated in the Terms of Accreditation, laboratories are required to report changes to
directorship, location, ownership, name, insolvency, or bankruptcy, and activity menu to the CAP
on an ongoing basis.
Changes in activity menu, including tests performed, scope of service, and analytical
methods can affect checklist usage or the selected requirements included in the
laboratory’s customized checklist. It is imperative that the laboratory notify the CAP
when changes occur. Submit test menu changes by logging onto cap.org
, e-LAB Solutions
Suite. Changes are submitted though Organization Profile.
Proficiency Testing Participation
Ongoing enrollment and successful participation in proficiency testing is required for maintaining
accreditation. Refer to the following sections for additional information:
• Accreditation Program Requirements for International Laboratories
,
• Proficiency Testing: Enrollment and Handling,
• Proficiency Testing (PT) Failure and Proficiency Testing Monitoring.
Self-inspection
At the beginning of the interim year of the two-year accreditation cycle, laboratories complete a
mandatory self-inspection, using the checklists sent to the laboratory for this purpose. The
laboratory must perform the self- inspection and return the Self-Inspection Verification Form
signed by the laboratory director within 60 calendar days after receiving the self-inspection
materials. The laboratory is required to correct all deficiencies cited and maintain records of
corrective action. The next CAP inspection team will verify that all such deficiencies have been
corrected. Deficiencies should be corrected within 30 days of the self-inspection, similar to the
correction of deficiencies cited by an inspection team. The laboratory must keep the self-
inspection records, including the findings and corrective actions on file for future reference.
Failure to perform the self-inspection is a serious deficiency and may result in an immediate on-
site inspection or revocation of accreditation.
Anniversary of Accreditation
Accreditation is maintained on a continuous basis provided that the laboratory continues to meet
the Terms of Accreditation. The CAP’s accreditation programs function on a fixed accreditation
cycle. This means that a laboratory will be inspected every two years within the three-month
complete the reapplication process online. The new checklists to be used in the inspection will
be sent to the laboratory when the reapplication is complete.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
85
CAP Reporting to Organizations and Other Government Agencies
The CAP’s accreditation programs are recognized by various organizations and government
agencies. As part of the recognition agreements, the CAP provides information on accredited
laboratories to those organizations and agencies, where applicable, such as copies of
inspection reports and other communications about the status of the laboratory’s CAP
accreditation or complaint investigations. The laboratory director’s signature on the Terms of
Accreditation form authorizes the CAP to provide accreditation information to the associated
agencies and organizations. The CAP’s accreditation program has a relationship with the
following accrediting organizations and government agencies:
• The Joint Commission
The Joint Commission accepts CAP accreditation of hospital laboratories. During the
hospital's Joint Commission survey, an administrative surveyor will routinely examine
laboratory safety and a physician surveyor will request and review information on the
performance improvement activities of the laboratory and its medical staff. Additionally, a
Joint Commission “tracer” investigation may intersect with the laboratory.
• The Centers for Medicare and Medicaid Services (CMS)
The CAP’s accreditation programs have been approved as a private accrediting
organization under CLIA by the CMS. Therefore, CAP-accredited laboratories may use
their CAP inspection in lieu of routine inspection by a CMS agent. This recognition
imposes the following obligations upon the CAP’s accreditation program:
o The CAP must ensure that laboratories are inspected every two years.
o The CAP checklist requirements must be at least as stringent as the CLIA
regulations.
o The CAP number assigned to an accredited laboratory corresponds to one and
only one CLIA certificate’s number.
• State Licensure
Some states license clinical laboratories. The CAP makes the results of the accreditation
decision available to state agencies upon request from the state agency.
The extent to which the CAP accreditation program is recognized by state governments
varies. The CAP has a formal recognition program with several states where CAP
accreditation can be used in lieu of a separate state inspection. The CAP has deeming
authority with the following states: California, Florida, Washington, Georgia and
Tennessee.
• Other Agencies/Organizations
The CAP also has reporting relationships with the following agencies/organizations:
o Department of Defense (DoD)
o Department of Veterans Affairs (VA)
o Society for Reproductive Assisted Technology (SART)
o United Network for Organ Sharing (UNOS)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
86
o National Marrow Donor Program (NMDP)
o Foundation for the Accreditation of Cellular Therapy (FACT).
CAP Website Resources
The CAP has a variety of tools that can be used to help stay current with changes to the CAP
accreditation programs and to manage laboratory information. Navigating the website using the
search functionality allows you to quickly find what you need. In addition to the items listed
below, laboratories may also refer to Appendix E
, CAP Accreditation Program Website Tools:
• e-LAB Solutions Suite
e-LAB Solutions Suite (eLSS) is the CAP’s online portal to manage accreditation
and proficiency testing. The portal provides helpful, convenient, and easy-to-use
tools to:
o Manage laboratory online access, user permissions, and individual profiles
o Manage laboratory accreditation documents, including customized accreditation
checklist and changes to activity menus
o Complete the application or reapplication
o Enter, review, and approve proficiency testing (PT) results with the interactive
online forms
o Connect to CAP Learning tools, assessments, and modules
o Access the Performance Analytics Dashboard
o View and print copies of evaluations, participant summary reports, kit
instructions, and result forms
o Access analyte scorecards, the customized PT shipping calendar, and other
analytical tools
o Access user guides and PT Exception Investigation Checklist tools
o Receive automated reporting email notifications with e-LAB Solutions Connect
(eg, proficiency testing data receipt)
o Access the library of past Focus on Compliance webinars.
• Performance Analytics Dashboard
The Performance Analytics Dashboard tool assists laboratories in managing risk and
compliance proactively. Updated daily, the dashboard gives laboratories a single
comprehensive view of all CAP proficiency testing results and accreditation information.
This complimentary tool delivers key insights to help identify and mitigate risk while
benchmarking laboratory performance. This tool is available to all CAP customers
through e-LAB Solutions Suite™.
• eAlerts
The CAP issues eAlerts as a means to communicate time-sensitive, critical, and
regulatory information. These may include significant changes to accreditation checklist
requirements, information to assist with interpreting requirements, and updates to the
inspection process. eAlerts are communicated by email and posted on the CAP website
under Laboratory Improvement, News and Updates.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
87
• My Profile
Personal demographic information is maintained through My Profile on the cap.org log-
in section. Individual users (eg, directors, supervisors, laboratorians, inspectors) can
create an account and update demographic information such as address, telephone,
and emails, as well as credentials and inspector availability.
• LAP Policies
The complete listing of all current Laboratory Accreditation Policies is available in e-LAB
Solutions Suite™ under CAP Accreditation Resources, Accreditation Manuals/
Retention Guidelines.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
88
NON-ROUTINE INSPECTIONS
Any on-site inspection performed in addition to the laboratory’s routine inspection is “non-routine”.
Non-routine inspections can be announced or unannounced. A non-routine inspection may be
required for the following reasons:
• Evidence of non-compliance with the Standards for Accreditation or accreditation
checklist requirements
• The need to confirm compliance with corrective actions taken after an inspection
• A complaint about the laboratory
• Repeated failures in proficiency testing
• Findings from a regulatory inspection
• The addition of a new discipline or sub-discipline
• Changes in directorship, ownership, or location
• Follow-up for a virtual inspection (if applicable).
The laboratory is ordinarily responsible for the cost of a non-routine inspection, with the
exception of a non-routine follow-up for virtual inspections, which is at no charge.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
89
COMPLAINTS AND INVESTIGATIONS
TOPIC
PAGE
Complaints
89
The
Complaint Process
89
CMS
Validation Inspections 90
Complaints
A complaint is any formal notification to the CAP or the discovery by the CAP of information
outside of the routine inspection process that raises the possibility of noncompliance with the
Standards for Accreditation and/or checklist requirements in a CAP-accredited laboratory or in
a laboratory seeking CAP accreditation.
The Complaint Process
Investigation begins as soon as CAP records a complaint. The investigation may include:
• A request for information from the laboratory
• A search of past inspection and proficiency testing results
• An unannounced, on-site inspection of the laboratory.
The CAP only investigates complaints related to the Standards for Accreditation and/or
accreditation checklist requirements. The CAP does not routinely investigate complaints such
as billing issues, human resource issues (eg, employee hiring practices) or result interpretation
as it relates to the general practice of pathology.
The CAP notifies the laboratory director of the complaint and communicates with the laboratory
director during the complaint resolution process. The complainant’s identity is kept confidential
and never released to the laboratory unless permission is obtained from the complainant.
Once information gathering is complete:
• The Complaints and Investigations Committee (CIC) will consider the evidence to
determine whether the basis for the complaint has been substantiated.
• The CI
C will determine what remedial actions, if any, need to be taken.
• The CAP’s Accreditation Committee will determine whether the facility will continue to
be accredited, be placed on probation or have its accreditation revoked.
The complaint will be closed as substantiated, not substantiated, not applicable or inconclusive.
At the conclusion of the complaint investigation, the CAP will send a letter to both the laboratory
director and the complainant (if contact information is provided), indicating that the CAP has

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
90
completed its investigation. The CAP is required to report all substantiated complaints, and/or
changes in accreditation status due to the complaint investigation to the appropriate state,
federal, or other oversight accreditation agencies.
CMS Validation Inspections
As part of the CAP’s approval for deeming authority as an accrediting organization for clinical
laboratories under the CLIA program, a percentage of CAP accreditation decisions are validated
by the Centers for Medicare or Medicaid Services (CMS) or its agents, or the state survey
agency. Validation ensures that the CAP inspection process continues to be equivalent to or
more stringent than the CMS laboratory survey. As a term of CAP accreditation, laboratories
must notify the CAP as soon as the facility finds itself to be the subject of a CMS validation
survey.
CMS validation inspections may occur either simultaneously with the CAP inspection or within
the 90-day timeframe following the CAP inspection. The CAP inspection team uses the CAP’s
inspection checklists; the CMS surveyor conducts the validation using the CLIA regulations.
Following a validation inspection, the laboratory receives a validation inspection report by mail
from the CMS surveyor and is asked to submit responses to the CMS following the instructions
provided to the laboratory. Laboratories must also submit to the CAP a copy of the responses
with the plan of correction for the deficiencies cited by the CMS including documentation that
demonstrates corrective action.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
91
ACCREDITATION PROGRAM REQUIREMENTS FOR INTERNATIONAL
LABORATORIES
TOPIC
PAGE
Special
Instructions for International Accreditation 91
Requirements
for International Laboratories 91
Proficiency Testing/External Quality Assurance 91
Laboratory Director 92
Laboratory Personnel 92
Scope of Disciplines 92
Test Volume 93
Limitations on Offering of Accreditation 93
Documentation Required in English 93
Resources
for International Laboratories 94
Accreditation
Fees and Charges 94
Inspection
Dates 94
Modified Inspection Process: Virtual Inspections
94
Inspection
Teams 95
Inspection
Team Travel 95
Inspection Report
95
Special Instructions for International Accreditation
The College of American Pathologists accredits clinical laboratories worldwide. The drive to
improve the quality of laboratory medicine and ultimately ensure better patient care is universal.
International laboratories with CLIA certificates must comply with both domestic regulations
(national, federal, state or provincial, and local) and US regulations.
Requirements for International Laboratories
Proficiency Testing/External Quality Assurance
International laboratories seeking CAP accreditation must:

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
92
• Enroll in CAP proficiency testing (PT) where CAP PT is available for a minimum of six
months prior to applying for accreditation
• Participate in PT on an ongoing basis and renew annually. Order by December 1
st
to
ensure full participation.
• International laboratories are required to enroll in CAP PT for all test/activities if a CAP PT
program is available.
• Contact the CAP on how to meet PT requirements if the PT product cannot be shipped
because of governmental restrictions or stability issues
• Obtain special permits or licenses for PT products that contain hazardous materials, such
as microbiology products. It is the laboratory’s responsibility to ensure the CAP receives
these permits prior to shipment. If the product cannot be released due to the lack of a
special permit or license, the laboratory will receive a zero (0%) score for that PT
shipment.
Laboratory Director:
The laboratory seeking accreditation must have a qualified laboratory director. The laboratory
director must be an MD, DO, or PhD, and meet all applicable qualifications defined in the CAP’s
Director Assessment Checklist requirement DRA.10100.
If the laboratory is subject to the CLIA regulations, and the laboratory director was educated
outside of the US, the laboratory must have records of an equivalency evaluation by a
recognized organization, such as the National Association Credential Evaluation Services, Inc.
(NACES) (http//:naces.org
) or the Association of International Credential Evaluators, Inc.
(AICE) (http://aice-eval.org).
Laboratory Personnel:
Supervisors and testing personnel must meet defined qualifications based on their role and the
complexity of testing performed.
• If the laboratory is subject to the CLIA regulations, and has personnel or supervisors
fulfilling a CLIA role (clinical consultant, technical consultant, technical supervisor, or
general supervisor) that were educated outside of the US, the laboratory must have
records showing that the credentials of these personnel are equivalent to those required
in the CLIA regulations. The equivalency evaluation must be performed by a recognized
organization, such as the National Association Credential Evaluation Services, Inc.
(NACES) (http//:naces.org
) or the Association of International Credential Evaluators,
Inc. (AICE) (http://aice-eval.org).
• Laboratories not subject to the CLIA regulations may authenticate educational
achievement according to prevailing governmental rules.
Scope of Disciplines:
All testing and disciplines performed by a laboratory in the same location must be listed in the
application. The CAP does not accredit portions of laboratories.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
93
Test Volume:
Laboratories are required to report test volumes in the online CAP accreditation application
as follows:
• International laboratories (including Canada) that have a CLIA certificate must:
o Report the test volume for moderate and high complexity testing performed on
patient specimens received from the US on the Non-waived section of the
application
o Report the non-waived and waived test volume for non-US citizen specimens in
the Waived section of the application.
• International laboratories that do not have a CLIA certificate must:
o Report the test volume for all testing complexities in the Total section of the
application.
Limitations on Offering of Accreditation:
The CAP may be unable to offer accreditation to laboratories in some geographic locations due
to country-specific risks such as US trade sanctions or a serious risk to inspector safety.
Documentation Required in English:
If English is not the operational language of your laboratory, the following requirements must be
met:
• Prior to scheduling an inspection, English translation of the following documents must be
available:
o Laboratory organization structure
o Instrument list
o Quality assurance/improvement programs
o Quality control programs
o Sample procedure for each laboratory discipline.
• If a US-based inspection team is requested, the laboratory must have staff conversant in
English or have interpreters available to support the inspection team with highly
specialized translations in the laboratory disciplines. The number of staff or interpreters
should be sufficient to accommodate each English-speaking inspector. The details on
the number of staff or interpreters needed should be discussed with the US-based Team
Leader prior to the inspection.
• Responses to any deficiencies cited at the inspection must be provided in English.
Certain supporting documentation to a deficiency may be in your native provided that the
key elements that demonstrate compliance are in English.
• Responses to any Proficiency Testing Compliance Notices (PTCN) must be available in
English. Supporting documentation to a PTCN may be in your native language provided
that the key elements that demonstrate compliance, as well as titles and major
headings, are in English.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
94
Resources for International Laboratories
More information and other resources are available online. To access this information, go to
cap.org
. Under the Laboratory Improvement heading, select International Laboratories.
Accreditation Fees and Charges
All fees and accreditation-associated charges (including international travel or non-routine
inspections) must be paid in full. Failure to do so will suspend any further advancement in the
accreditation process, including issuance of accreditation.
Inspection Dates
Inspections of international laboratories are conducted as announced inspections because of
logistical challenges and the common requirement for travel visas. Therefore, inspection dates
for international laboratories are arranged, and the laboratory is aware of the scheduled date.
• The inspection team leader will contact the laboratory director(s) within two weeks of
receiving the Inspector’s Inspection Packet to schedule the date.
• The inspection must occur no more than 90 calendar days before the laboratory’s
anniversary date for routine inspection. A mutually acceptable date is preferable, but in
the case of disagreement, the inspection is scheduled at the convenience of the
inspector.
• International laboratories not subject to CLIA regulations may undergo inspection
beyond the three-month timeframe after testing commences to accommodate
scheduling multiple inspections in the same country or region.
• The inspection team leader will send a letter to the laboratory director indicating the
inspection date, projected schedule, team member listing, any special requests, and
documentation instructions.
Since the inspections are scheduled in advance, inspection teams are not required to notify the
laboratory one-hour prior to arrival at the laboratory.
Modified Inspection Process: Virtual Inspections
In response to the global health emergency and associated travel restrictions, the CAP has
modified the laboratory inspection processes to allow international inspections at laboratories
not subject to the CLIA regulations to be virtual instead of in-person. This is a temporary
process implemented in response to the global pandemic. Because the duration of the
pandemic and associated travel restrictions is unknown, we recommend that accredited
international laboratories continue to budget for their inspection expenses, including airfare for
US inspectors. If either a) an on-site inspection is required based on the findings from the virtual
inspection, or b) the international laboratory has a CLIA license where an on-site inspection is
required, the laboratory will be billed for the airfare expense. For additional information about
virtual inspections go to cap.org and use the search function for “virtual inspections”.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
95
Inspection Teams
Most Team Leaders for inspecting international laboratories are US-based. The team leader
will include appropriate qualified international inspectors as team members whenever possible
and practical.
To be considered for an inspection team, inspectors based outside the US must have
successfully completed training and maintain competency as a team leader or member.
Likewise, the inspector must be currently or previously affiliated with a CAP-accredited
laboratory.
Regional team member assignments are made from the same country or geographic region as
the laboratory being inspected. Exceptions may be made with the approval of the chair of the
Council on Accreditation or the CAP vice president for the CAP Accreditation Programs.
Requests for exceptions should be submitted as early as possible.
Inspection Team Travel
Flights requiring airline travel are arranged based on the following:
• Inspectors originating in the US will:
o Travel business class for all flight legs to countries outside of North America
o Travel economy class for flights within the US or within destination country.
• Inspectors originating outside of the US will:
o Travel business class for all portions of flights with total flight time exceeding five
hours (excluding layovers)
o Travel economy class for flights of five hours or less.
• US Department of Defense contract laboratory inspections will:
o Travel economy class (the CAP Travel Desk books premium upgradable economy
class airfare to allow inspectors the option to personally upgrade their tickets).
Inspector’s Summation Report
International inspections are often grouped in tours to control costs for the participating
laboratories. More than one laboratory in a country or region may be inspected in a short time
span by one inspection team. The team leader must return the Inspector’s Summation Report
(ISR) to the CAP within two days of the inspector’s return to the US. (Refer to
After the
Inspection: Inspection Team for shipping instructions).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
96
Appendix A:
Accreditation Checklists Overview
This appendix includes a complete listing of the accreditation checklists along with a brief
description and notes relating to the usage of each checklist based on the September 22, 2021
Checklist edition. It does not include all possible uses for a particular checklist. Refer to the
Accreditation Checklists section for more information on checklist components and accessing
the checklists via cap.org
. For questions about checklist usage, contact the CAP at 800-323-
4040 or 847-832-7000.
All Common Checklist (COM)
•
Proficiency testing
•
Policy and procedure manuals
•
Specimen collection and handling
•
Quality management
•
Reporting of results
• Reagents
•
Instruments and equipment maintenance/function checks
•
Thermometers and temperature dependent equipment and environments
•
Pipettes and analytic balances
• Waived test implementation
•
Test method validation/verification-nonwaived tests
•
Individualized quality control plan.
NOTE: The COM Checklist is used in conjunction with the discipline-specific checklists (eg,
Anatomic Pathology, Chemistry and Toxicology) to inspect each laboratory section.
Anatomic Pathology Checklist (ANP)
•
Surgical pathology
•
Intraoperative consultation
•
Fine-needle aspiration (FNA)
•
Histology
•
Immunohistochemistry and immunofluorescence microscopy
•
In situ hybridization (ISH)
•
Predictive marker testing
•
Digital image analysis
•
Circulating tumor cell analysis
•
Flow cytometry data interpretation
•
Autopsy pathology
• Forensic autopsy

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
97
•
Electronic microscopy
•
In vivo and ex vivo microscopy.
NOTE: If FNAs are screened by a cytotechnologist, the Cytopathology Checklist must be used
for inspection. If the technical component of flow cytometry is performed at the laboratory, the
Flow Cytometry Checklist must be used for inspection.
Biorepository Checklist (BAP)
• Quality management
• Biospecimen collection and handling
• Biospecimen processing and quality, including DNA/RNA extraction/amplification, cell
fractionization, cell and tissue culture, and histology
• Specialized techniques, such as whole slide imaging, digital image, tissue microarray,
laser capture microdissection, and molecular methods
• Inventory management systems
• Storage
• Source and sponsor facilities
• Informed consent and institutional review board
• Distribution policies and agreements.
NOTE: The BAP Checklist is only for facilities enrolled in the Biorepository Accreditation
Program.
Chemistry and Toxicology Checklist (CHM)
•
Automated chemistry procedures
•
Blood gas analysis
•
Therapeutic drug monitoring
•
Toxicology screening and confirmatory testing
•
Prenatal screening
•
Cystic fibrosis sweat testing
•
Tumor marker, immune system, and infectious disease immunoassays
•
Hemoglobin separation
• Methods, such as thin layer chromatography (TLC), gas chromatography (GC), high
performance liquid chromatography (HPLC), mass spectrometry (MS), Imaging MS,
radioimmunoassay (RIA), and electrophoresis.
Clinical Biochemical Genetics Checklist (CBG)
•
Diagnostic testing for inborn errors of metabolism
• Methods such as enzyme assays, TLC, GC, HPLC, MS, electrophoresis, and RIA
•
Newborn screening.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
98
Cytogenetics Checklist (CYG)
•
Cytogenetic studies for constitutional and neoplastic disorders
•
ISH, including predictive marker testing
•
Digital image analysis
•
Genomic copy number analysis using microarray.
Cytopathology Checklist (CYP)
• Gynecologic cytopathology
• Non-gynecologic cytopathology, including fine-needle aspiration
• Cytology processing and staining
• Immunocytochemistry staining, including predictive marker testing
• Cytology screening, manual and automated.
NOTE: Laboratories that do histology processing of cell blocks and tissues must be inspected
with the Anatomic Pathology Checklist.
Director Assessment Checklist (DRA)
•
Laboratory director qualifications
•
Laboratory director responsibilities.
NOTE: One copy of the Director Assessment Checklist is provided to the team leader for each
laboratory inspected.
Flow Cytometry Checklist (FLO)
•
Blood lymphocyte subset enumeration
•
CD34 stem cell enumeration
•
Leukemia and lymphoma immunophenotyping
•
DNA content and cell cycle analysis
• Rare event flow cytometric analysis.
Forensic Drug Testing Checklist (FDT)
•
Nonmedical drug testing
•
Screening and confirmatory testing for different specimen types (urine, blood, oral
fluid, hair, meconium, umbilical cord, and nail)
•
Specimen handling and chain of custody
•
Certification/inspection of results
•
Methods, such as immunoassays, LC, GC, and MS.
NOTE: The FDT Checklist is only for laboratories enrolled in the Forensic Drug Testing
Program.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
99
Hematology and Coagulation Checklist (HEM)
•
CBC and differentials, automated and manual
•
Reticulocytes, automated and manual
•
Bone marrow preparations
•
Abnormal hemoglobin detection
•
Blood film examination for microorganisms
•
Body fluid cell counts (automated and manual) and differentials
•
Semen analysis, automated and manual
•
Routine coagulation assays
•
Specialized coagulation assays, including factor assays, mixing studies, D-dimer,
electrophoresis studies, and platelet function assays.
Histocompatibility Checklist (HSC)
•
HLA testing by serologic, molecular, flow cytometry, immunoassay, and
solid phase methods
•
Class I and II antigen typing
•
HLA antibody screening, identification, and crossmatching
•
DNA typing, including low and high resolution typing, and DNA sequence-based typing
•
Donor-recipient histocompatibility, including renal, stem cell, and nonrenal
organ transplants
•
Hematopoietic progenitor cell engraftment monitoring.
NOTE: Laboratories performing HLA testing by next-generation sequencing must also use the
Molecular Pathology Checklist for inspection.
Immunology Checklist (IMM)
•
General immunology assays, manual and automated
•
Immune system profiles
•
Tumor marker and infectious disease immunoassays
•
Microbial antigen testing
•
Waived molecular-based microbiology tests
•
ABO/Rh and antibody screening (non-transfusion-related)
•
Syphilis serology
•
Western blot.
Laboratory General Checklist (GEN)
•
Quality management system
•
Specimen collection
•
Chain-of-custody specimen collection and handling
•
Direct-to-consumer testing
•
Specimen transport and tracking
•
Result reporting

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
100
•
Quality of water
•
Laboratory computer services
•
Telepathology and remote data assessment
•
Whole slide imaging
•
Personnel
•
Physical facilities
•
Laboratory safety
•
California laboratory licensure requirements.
NOTE: A Laboratory General Checklist is provided for inspections of all laboratories and
biorepositories.
Limited Service Laboratory Checklist (LSV)
•
Automated and manual hematology testing, including CBC, reticulocytes, and
differentials
•
Routine coagulation assays
•
Body fluid analysis, including semen analysis
•
Automated general chemistry
•
Blood gas analysis
•
Therapeutic drug monitoring
•
Screening for drugs of abuse
•
Tumor marker and infectious disease immunoassays
•
Urinalysis dipstick and microscopy, manual and automated methods
•
Microbiology specimen setup, direct specimen examination, stains, and antigen
typing for various subdisciplines
•
General immunology assays, including immune system profiles and microbial
antigen/antibody testing, non-transfusion-related immunohematology testing, and
syphilis serology
•
Microbial antigen/antibody testing
•
Non-transfusion-related immunohematology testing
•
Syphilis serology
•
Waived molecular-based microbiology tests.
NOTE: The LSV Checklist is used to inspect freestanding laboratories or a section of a
laboratory doing a limited number of basic tests in multiple disciplines (eg, outpatient or “STAT”
laboratories). It is made up of a limited subset of requirements from other checklists to reduce
the burden of using multiple checklists when a laboratory’s scope of services is confined to the
most commonly performed tests. The All Common, Laboratory General, and Director
Assessment Checklists are used along with the LSV Checklist for inspections. It is not
appropriate for single-discipline or specialized laboratories; such laboratories must use the
relevant discipline-specific checklist(s).
The ability of a laboratory to use the LSV Checklist is determined by a laboratory’s
Accreditation Unit Activity Menu. Laboratories with activities extending beyond the scope of the

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
101
LSV Checklist must use the discipline-specific checklists. The LSV Checklist does not cover
the following services:
• Hematology — bone marrow evaluation, blood film examination for microorganisms, and
abnormal hemoglobin detection (except the sickling test)
• Coagulation — factor assays, mixing studies, electrophoresis studies, and platelet
function testing
• Chemistry — toxicology (other than drug of abuse screening and serum or whole blood
alcohol), mass spectrophotometry, electrophoresis, chromatography, AFP, RIA, and
sweat testing for cystic fibrosis
• Microbiology — cultures beyond initial plating, susceptibility testing, mycology other than
KOH or wet preps, mycobacteriology, parasitology other than pinworm preparations,
virology, and nonwaived molecular microbiology
• Transfusion medicine — pretransfusion testing, antibody identification, blood storage
and issue
• Testing in the disciplines of anatomic pathology, clinical biochemical genetics,
cytopathology, cytogenetics, histocompatibility, flow cytometry, molecular pathology,
and point-of-care-testing.
Microbiology Checklist (MIC)
•
Culture setup, staining, antigen typing, screening, identification, and susceptibility
testing for bacteriology, mycology, mycobacteriology, and virology
•
Parasitology, including stool for ova and parasites and blood film examination for
microorganisms
•
Molecular microbiology, including waived and nonwaived FDA-cleared/approved
methods, modified methods, and laboratory-developed methods
•
Microbial identification, using methods, such as MALDI-TOF MS, GC, HPLC, ISH,
target and signal amplification, and sequencing.
NOTE: Laboratories performing molecular infectious disease testing by next-generation
sequencing must also use the Molecular Pathology Checklist for inspection.
Molecular Pathology Checklist (MOL)
•
Clinical molecular genetics testing, including oncology
, inherited
disease,
pharmacogenomics, HLA tying, relationship testing, and forensic identity applications
•
Molecular assay validation
•
Methods, such as electrophoresis, PCR, arrays, in situ hybridization, digital image
analysis, and sequencing
•
Next-generation sequencing, including noninvasive screening of maternal plasma to
detect fetal aneuploidy
•
Stem cell engraftment monitoring.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
102
Point-of-Care Testing Checklist (POC)
• Tests performed at or near the patient bedside (nondedicated space)
•
Kit tests or hand-carried instruments (or otherwise transported to the patient
location)
•
Waived and moderate-complexity testing
• Modified FDA-cleared/approved POCT
• Blood gas analysis
•
D-dimer studies
•
Waived molecular-based microbiology testing
•
Provider-performed microscopy.
NOTE: The POC Checklist is used for inspection of testing performed at or near the site where
the patient is located only (with non-dedicated space). It contains a subset of requirements
found in other checklists. A discipline-specific checklist(s) may be required in addition to the
POC Checklist if certain analytes warrant its use. Laboratories with fixed dedicated testing
space require either a Limited Service Laboratory Checklist or additional discipline-specific
checklist(s).
A separate checklist must be completed for each POCT location when POCT records are not
maintained in a central location by a designated POCT coordinator.
Reproductive Laboratory Checklist (RLM)
•
Complete semen analysis, automated and manual methods
•
Biochemical testing
•
Anti-sperm antibody testing
•
Sperm processing for therapeutic insemination
• Embryology procedures
•
Embryo and gamete cryopreservation
•
Donor reproductive cell/tissue programs.
NOTE: The RLM Checklist is only for laboratories enrolled in the Reproductive Laboratory
Accreditation Program.
Transfusion Medicine Checklist (TRM)
•
Immunohematology testing, manual and automated
•
Compatibility testing, including computer crossmatches
•
Perinatal testing
•
Transfusion procedures and adverse reactions
•
Therapeutic phlebotomy
•
Donor and therapeutic apheresis
•
Component preparation, storage, and modification

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
103
•
Cellular therapy
•
Tissue storage and issue
•
Donor selection, collection, and testing.
NOTE: Laboratories with immunohematology testing limited to ABO, Rh, antibody screens (non-
transfusion), and direct antiglobulin testing may be inspected with the Immunology Checklist.
Urinalysis Checklist (URN)
• Urinalysis dipstick, automated and manual methods
•
Manual urine microscopy
•
Automated microscopy systems.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
104
Appendix B:
Instructions for Determining Test Volume
These instructions are provided to assist CAP-accredited laboratories in calculating and
reporting annual test volumes for laboratory sections/departments in Organization Profile on
cap.org. The CAP uses this data for inspection planning and laboratory monitoring. The CAP
also reports this data annually to the Centers for Medicare and Medicaid Services (CMS) for
laboratories that are subject to US regulations.
The following types of test volume data is requested based on laboratory type:
Laboratory Type
Non-Waived
Waived
Total Volume
Laboratories subject to US
regulations (CLIA, VA, or CLIP
certificate)
X * X
Laboratories not subject to US
regulations
X
Non-waived — Includes high and moderate-complexity testing Do not include calculations (eg,
A/G ratio, MCH, base excess, anion gap, iron saturation, INR), quality control, quality
assurance, proficiency testing assays, and tests sent to referral laboratories.
* Laboratories located outside of the US with a CLIA certificate must only include non-
waived test volume for specimens received from the US or its territories. Test volumes for all
other testing must be included under the waived category.
Waived — Includes waived testing and other tests or procedures to be inspected that are not
classified by the CMS (eg, autopsy, employee drug testing, assisted reproductive technology-
related procedures).
Total Volume – Includes test volumes for all testing complexities and procedures not classified
by the CMS.
Specialty/Discipline information:
Specialty/Discipline
Instructions for Determining Test Volume
Chemistry
• Count each non-calculated analyte (eg, a l ipid panel consisting of a
total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides
equals four tests)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
105
Cytogenetics
• Count the number of tests by the number of specimen types processed
on each patient (eg, a bone marrow and a venous blood specimen
received on the same patient equal two tests)
•
Count each special stain ordered and reported as a separate test
NOTE: For all other genetic tests, the number of tests is determined by
the number of results reported in the final report.
Cytology
• For manual gynecologic and non-gynecologic cytology, count each
slide (not each case) as one test for both Pap tests and non-
gynecologic cytology
•
For non-gynecologic slide preparations made using liquid-based slide
preparatory techniques that result in cell dispersion over one-half or
less of the total available slide, count as one-half slide
•
Refer to the manufacturer’s product insert to determine how to count
test volume for gynecologic slides screened by automated devices
when only a portion of the slide is reviewed
Flow Cytometry
• Count each measured individual analyte (eg, T cells, B cells, CD4)
that is ordered and reported separately
Hematology
• Count each measured
analyte of a complete blood count, coagulation
profile, and body fluid analysis that is ordered and reported
separately
•
Count white blood cell differentials as one test
Histocompatibility
• Count each HLA typing, each HLA antibody screen, or each HLA
crossmatch as one test (eg, a B-cell crossmatch, a T-cell crossmatch,
and an auto-crossmatch between the same donor and recipient pair
equals three tests)
•
Count each disease-associated antigen test result (eg, HLA-B27) as
one test
Histopathology
• Count each block (not slide) as one test
•
Add the number of special stains performed on histology slides to the
total number of specimen blocks prepared by the laboratory
•
Do not include autopsy services under the non-waived test volume
Immunohematology
• Count ABO, Rh, antibody screen, crossmatch, direct antiglobulin test,
and antibody identification as separate tests
Immunology
• Count testing for allergens as one test for each allergen

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
106
Microbiology
• Count susceptibility testing as one test for each group of antibiotics
used to determine sensitivity for one organism
•
Count cultures as one test request from each specimen regardless of
the extent of identification, number of organisms isolated, and the
number of tests/procedures required for identification
•
Count molecular multiplex panels as one per test request from each
specimen regardless of the number of organisms identified
•
Count each Gram stain or acid-fast bacteria (AFB) smear requested
from the primary source as one test
•
Count each parasitology test request for each specimen regardless of
the number of tests/procedures required for identification as one test
Example
: if the order for a sputum specimen includes a routine
bacteriology culture, Gram stain, a mycology test,
an AFB smear, and an
AFB
culture, this would equal five tests. A stool parasitology direct smear,
concentration technique, and review of the prepared slide are collectively
counted as one test.
Molecular Pathology
• Count each genetic test ordered and reported as one test
•
Count each next-generation sequencing test ordered with one report
(eg, a gene panel, exome or genome) as one test
Point-of-Care Testing
• Count point-of-care (POC) testing according to the specialty of the test
•
Count non-waived and waived test volumes separately (for
laboratories subject to US regulations)
Example: If blood gas testing is done as part of POC testing, count it the
same as if it were done in a chemistry department. Similarly, a
macroscopic (dipstick) urinalysis test done as part of POC shou
ld follow
the urinalysis criteria listed below.
Reproductive
Laboratory Medicine
• Count each test in a diagnostic semen analysis panel (eg, sperm
count, motility, morphology) as separate non-waived tests
•
Include volume for sperm preparation for insemination and assisted
reproductive technology-related procedures (number of cycles, fresh
and frozen) under waived testing
Urinalysis
• Count macroscopic and microscopic examinations as separate tests
•
Count dipstick testing as one test, regardless of the number of reagent
pads on the strip
Reference: Clinical Laboratory Improvement Amendments (CLIA) Application for Certification,
Guidelines for Counting Tests for CLIA; Form CMS-116;
https://www.cms.gov/Medicare/CMS-
Forms/CMS-Forms/Downloads/CMS116.pdf.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
107
Appendix C:
Minimum Period of Retention of Laboratory Records and Materials
(CAP Policy PP)
The College of American Pathologists (CAP) makes the following recommendations for the
minimum requirements for the retention of laboratory records and materials. They meet or
exceed the regulatory requirements specified in the Clinical Laboratory Improvement
Amendments of 1988 (CLIA 88). It may be appropriate for laboratories to retain records and/or
materials for a longer period of time when required for patient care, education, quality
improvement, medical/legal, or other needs, or if required by institutional policy. Some state
regulations as well as federal mandates may require retention of records and/or materials for a
longer time period than that specified in the CLIA 88 regulations. Therefore, any applicable
national, federal, state (or provincial) or local laws should be reviewed carefully when individual
laboratories develop their record retention policies.
Material/Record
Period of Retention
GENERAL LABORATORY
Accession records
2 years
Specimen requisitions (including the patient chart or
medical record if used as the requisition)
2 years
Chain-of-custody collection, receipt, accessioning,
and handling records
2 years (or longer as
applicable)
Quality management records
2 years
Instrument/equipment maintenance and function
check records (including temperature charts)
2 years
Proficiency testing records
2 years
Policies and procedures 2 years following
discontinuance
Test method validation/verification records (method
performance specifications)
Length of time the test
is in use, plus 2
additional years
Quality control records
2 years
Individualized Quality Control Plan (IQCP), including
risk assessment and supporting data, and approval of
quality control plan
Length of time the test
is in use, plus 2
additional years
following
discontinuation of the
IQCP
Ongoing IQCP quality assessment data
2 years
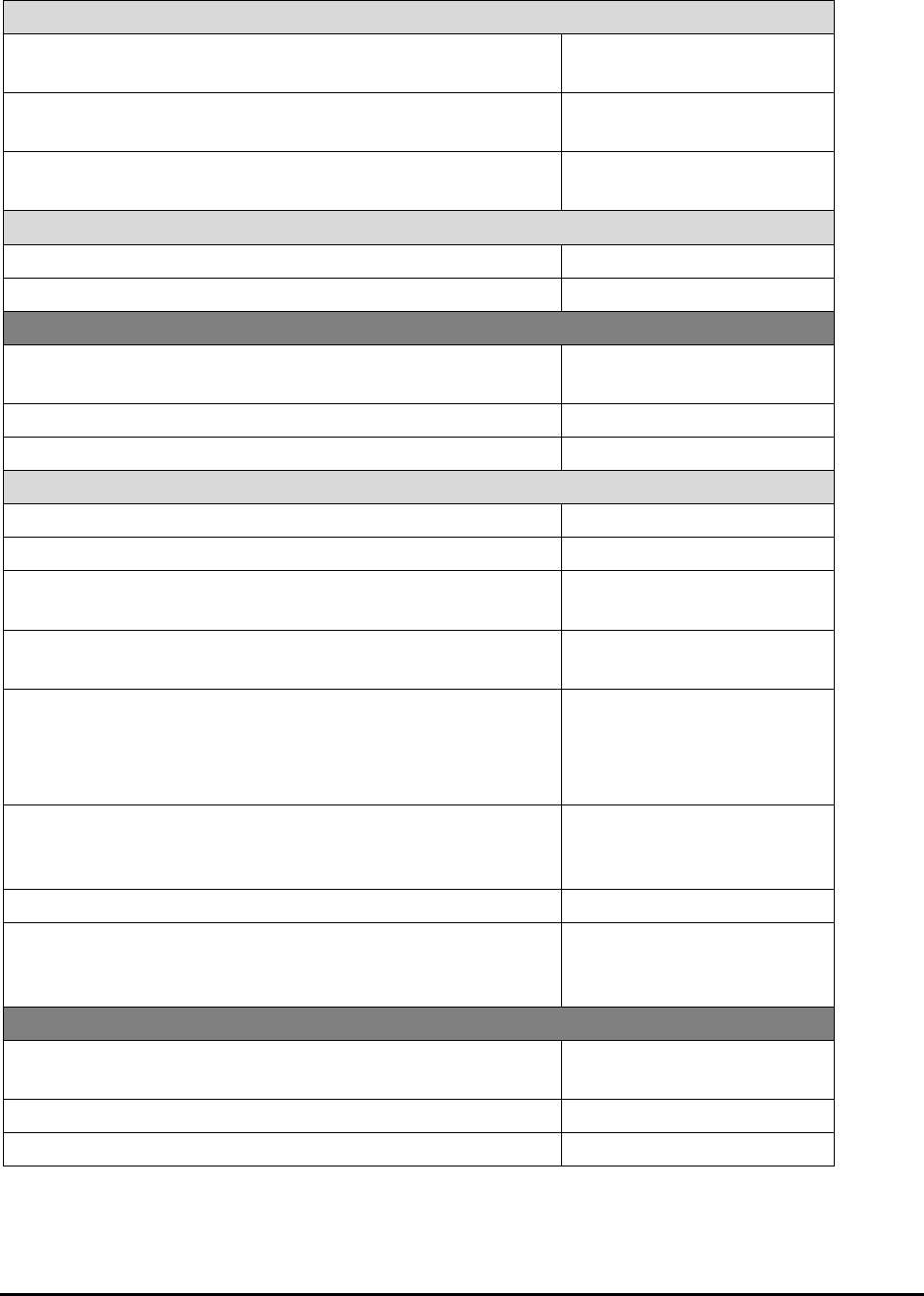
College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
108
Laboratory Computer Services
Computer system validation records 2 years beyond the life
of the system
Records of changes to software, the test library, and
major functions of laboratory information systems
2 years beyond the life
of the system
Ongoing computer system checks (eg, calculation
verification)
2 years
Personnel Records
Competency assessment records
2 years
Training records
2 years
SURGICAL PATHOLOGY (including bone marrows)
Wet tissue 2 weeks after final
report
Paraffin blocks (including cell blocks)
10 years
Reports
10 years
Slides
Immunohistochemistry batch control slides
2 years
Surgical pathology slides
10 years
Bone marrows slides with associated peripheral
blood smear(s) included in the bone marrow report
10 years
Fluorochrome-stained slides At the discretion of the
laboratory director
In situ hybridization images (refer to Note 1) or
permanent slides
10 years - Neoplastic
disorders
20 years -
Constitutional disorders
Digital images used for primary diagnosis 10 years (if original
glass slides are not
available)
Digital images for Circulating Tumor Cells
10 years
Datasets from ex-vivo microscopy (EVM) or in-vivo
microscopy (IVM) systems used to aid in
interpretation or diagnosis
10 years (data must be
retrievable for this
period)
ELECTRON MICROSCOPY
Wet tissue 2 weeks after final
report
Resin blocks
10 years
Pictures and reports
10 years

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
109
CYTOLOGY
Reports
10 years
Slides
Immunochemistry batch control slides
2 years
Gynecologic cytology glass slides
5 years
Non-gynecologic cytology glass slides (including fine
needle aspiration (FNA) slides
10 years
NON-FORENSIC AUTOPSY
Wet tissue 3 months after final
report
Paraffin blocks
10 years
(refer to ANP.12500 for
further detail)
Slides
10 years
Reports
10 years
Autopsy consent
10 years
FORENSIC AUTOPSY
Wet stock tissue
1 year
Paraffin blocks
10 years
Reports
Indefinitely
Slides
50 years or
30 years if a DNA
sample is available
Gross photographs/images
Indefinitely
Accession records
Indefinitely
Body fluids and tissues for toxicology
1 year
Representative sample suitable for DNA Analysis
Indefinitely
Body transfer and disposition records
Indefinitely
CLINICAL PATHOLOGY
Testing Records
Instrument printouts (not interfaced with laboratory
computer system) and worksheets
2 years
Patient test results and reports, including original and
corrected reports, and referral laboratory reports
2 years
Direct-to-consumer testing results, including
reference intervals
10 years

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
110
Patient Specimens
Serum and plasma 48 hours; exceptions
may be made at the
discretion of the
laboratory director.
Longer storage
requirements may be
necessary for patients
admitted for suspected
drug overdoses.
Citrated plasma At the discretion of the
laboratory director (see
HEM.36940)
CSF and body fluids (except urine)
48 hours
Whole blood specimens, including blood gas
specimens
At the discretion of the
laboratory director
Urine 24 hours; exceptions
may be made at the
discretion of the
laboratory director
Clinical Pathology Slides
Peripheral blood films
7 days
Permanently stained body fluid slides
7 days
Permanently stained microbiology slides prepared
from clinical specimens (including blood culture
bottles)
7 days
CYTOGENETICS
Final reports
10 years - neoplastic
disorders
20 years -
constitutional disorders
Images (in situ hybridization (ISH) or non-ISH) (refer to
NOTE 1)
10 years - neoplastic
disorders
20 years -
constitutional disorders
Chromosomal microarray data
Original scan
2 weeks after the final
report is released
Sufficient original data to support primary results
generated and re-analysis
2 years

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
111
Slides
Permanently stained slides
3 years
Fluorochrome stained slides At the discretion of the
laboratory director
Chromosomal microarray slides At the discretion of the
laboratory director
Original specimens and cultures Until release of the final
report
Processed specimens or cell pellets 2 weeks after final
report
MOLECULAR PATHOLOGY
Fluorochrome stained slides
At the discretion of the
laboratory director
Chromosomal array slides At the discretion of the
laboratory director
In situ hybridization images* (see Note 1) or
permanent slides
10 years - neoplastic
disorders
20 years -
constitutional disorders
Reports
10 years - neoplastic
disorders
20 years -
constitutional disorders
Next Generation Sequencing Data
Sequence read files (eg, FASTQ, uBAM, BAM,
CRAM) and variant calling files (eg, VDF, gVCF)
2 years
Array Data
Original scan 2 weeks after the final
report is released
Sufficient original data to support primary results
generated and re-analysis
2 years
FLOW CYTOMETRY
Data for evaluation of hematolymphoid neoplasia,
PNH, and congenital immunodeficiency
10 years
Data for routine lymphocyte subset and CD34+
enumeration
2 years
TRANSFUSION MEDICINE
Policies and procedures, including approval, review,
and discontinuance
5 years

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
112
Quality Management Records
Proficiency testing records
5 years
Management reviews for the effectiveness of the
quality system
5 years
Blood supplier agreements
5 years
Irradiation dose delivery
5 years
Control systems for patient testing
10 years
Control systems for donor testing
10 years
Instrument and equipment maintenance and function
checks
10 years
Temperature monitoring (eg, graphs, logs) of
refrigerators, freezers, and platelet incubator
10 years
Inspections of blood/critical materials
10 years
Inspection of weld for completeness
10 years
Specimens
Patient pretransfusion testing specimens
7 days post-transfusion
Specimens from blood donor units
7 days post-transfusion
Patient Records
Orders and requests for blood/blood components
5 years
Transfusion administration records
10 years
Final unit disposition
10 years
Patient pre-transfusion testing results/interpretation
10 years
Immediate evaluation/interpretation of transfusion
reactions
10 years
Final inspection and verification of blood before issue
10 years
Evaluation/interpretation of delayed transfusion
reactions
10 years
Emergency release of blood, including signature of
requesting physician
10 years
Therapeutic phlebotomy/apheresis records
10 years
Transfusion problems such as transfusion reactions,
unexpected antibodies, and special transfusion
requirements.
Indefinitely
Donor Records
Blood/component donor information, consent and
collection
10 years
Donor blood testing
10 years

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
113
Retyping of donor units
10 years
Donor notification of significant findings
10 years
Component production
10 years
Look back investigation/disease reporting
10 years
Final unit disposition
10 years
Irradiation of cellular components
10 years
Acceptability of returned units into inventory
10 years
Indefinitely and permanently deferred donors
Indefinitely
Donors placed under surveillance (for recipient
protection)
Indefinitely
Personnel
Competency records
5 years
Training records
†
(see Note 2)
5 years
Records of employee signatures, initials,
identification codes, and inclusive dates of
employment
10 years
Other Records
Identification of individuals performing each
significant step in collection, processing, compatibility
testing, and transportation of blood and blood
components
10 years
Traceability of blood, blood components and critical
materials
10 years
Container qualification/process validations
10 years
Tissue Records (including hematopoietic progenitor cells)
Daily temperature monitoring
10 years
Investigation of adverse events
10 years
Discontinued policies, procedures and other
controlled documents
10 years
Collection, transportation, processing, issuing, and
disposition
10 years beyond
tissue’s disposition or
expiration, whichever is
longest
* Note 1: There is no retention requirement for images of glass slide preparations when the
source slides remain readable for the required retention period. A scanned image may not
take the place of the source slides.
†
Note 2: The five-year retention requirement for transfusion medicine training records aligns
with the AABB Standards to provide consistency for laboratories that have coordinated
CAP/AABB inspections and for labs in states that are required to follow AABB regulations

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
114
(eg, California). Reference Table 6.2C in the AABB Standard lists: Standard 2.1.2 - Training
Records of Personnel – minimum retention time (in years) – 5.
REFERENCES
1. Department of Health and Human Services, Centers for Medicare and Medicaid Services.
Clinical laboratory improvement amendments of 1988; final rule. Fed Register. 2003(Jan 24):
[42CFR493.1105].
2. Food and Drug Administration. Current good manufacturing practice for blood and blood
components. Records and reports. Records. Washington, DC: US Government Printing Office,
1999(Apr 1): [21CFR606.160].
3. Fanaoff J. Retention of Pediatric Medical Records. American Academy of Pediatrics. March
2016. https://www.aap.org/en-us/professional-resources/practice-transformation/managing-
practice/Pages/Retention-of-Pediatric-Medical-Records.aspx. Accessed April 15, 2019.
Document ID: Retention of Laboratory Records
and Materials
Document type: Public Policy
Responsible: Council on Scientific Affairs
Approve: Board of Governors
Consult: None
Inform: CAP Policy Manual; CAP Website
Adopted August 1995
Revised September 1995
Reaffirmed August 1998
Revised November 2000
Revised May 2001
Revised November 2001
Revised November 2004
Revised November 2005
Revised August 2007
Revised March 2010
Reaffirmed June 2013
Revised September 2016
Revised September 2020
Revised January 2021

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
115
Appendix D: Glossary of Terms
Additional definitions are found in the Accreditation Checklists.
Accreditation
The determination by the CAP that a laboratory or biorepository has successfully met the CAP’s
accreditation program Standards.
Accreditation Checklist
A detailed series of requirements designed to evaluate whether the laboratory or biorepository meets
the standards set forth in the CAP’s accreditation program Standards. Each checklist serves as a tool
to guide the conduct of the inspection.
Accreditation Cycle
The sequence of events for laboratories spanning a two-year period that leads to an accreditation
decision.
Accreditation Packet
The packet of information that is sent to a laboratory following a decision to grant accreditation. The
packet contains a certificate of accreditation, CAP letter of accreditation, final list of deficiencies, and a
press release.
Accreditation Unit (AU)
The laboratory, department, or other organizational unit that is evaluated and can receive
accreditation. An AU usually has a unique CLIA number, is located in one building or campus, and
falls under the leadership of a single laboratory director who is named on the CLIA certificate.
Accreditation Unit Activity Menu (Laboratory-Specific Activity Menu)
The list of tests and non-test activities specific to a laboratory. The AU-specific activity menu is used
to create the customized checklists, monitor PT, inspect, and report accreditation.
Accreditation with Requirements
Accreditation status assigned to a laboratory that is able to demonstrate compliance with all
accreditation requirements and all required corrective action, and supporting documentation indicates
compliance with the applicable CAP’s Standards for Accreditation; however, during the review
process, a need has been identified to monitor ongoing compliance through a non-routine inspection,
the submission of documents, or both.
Activity
A reportable assay (eg, glucose, serum), scope of service (eg, therapeutic drug monitoring), or
analytic method (eg, dipstick, manual).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
116
Activity Menu, Master
The list of all tests and non-test activities subject to inspection and accreditation.
Alternative Performance Assessment
A system for determining the reliability of laboratory examinations for which no commercial proficiency
testing products are available, are not appropriate for the method or patient population served by the
laboratory, or participation is not required by the accrediting organization.
Anatomic Pathology
The major branch of pathology dealing with gross, microscopic, and molecular alterations in tissues
and cells. Anatomic pathology includes, but is not limited to, autopsy pathology, surgical pathology,
cytopathology, related aspects of molecular pathology, and the laboratories providing service in these
areas.
Anniversary Date
The fixed date at which the laboratory accreditation will terminate unless the laboratory reapplies or
(under some circumstances) is in the process of accreditation. The anniversary date is fixed and
biennial (occurring every two years).
Authority
The power or right to give orders, make decisions, direct someone, or control a process.
BAP
See Biorepository Accreditation Program.
Biorepository
A facility that collects, processes, stores, and distributes biospecimens for research.
Biorepository Accreditation Program (BAP)
A CAP accreditation program that accredits facilities that collect, process, store, and distribute
biospecimens for research.
CAP 15189
• CAP 15189 is a voluntary, nonregulated program providing accreditation to the ISO
15189:2012 Standard as published by the International Organization of Standardization.
• CAP 15189 assesses a laboratory’s quality management system to include all facets of
laboratory management, technical testing, and interacting departments.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
117
• CAP 15189 is a highly disciplined approach to implementing a quality management system,
sustaining continual improvement and evaluating the laboratory’s effectiveness and
contribution to the quality of patient care.
• CAP 15189 does not replace the CAP’s CLIA-based Laboratory Accreditation Program, but
rather complements CAP accreditation and other quality systems.
CAP-accepted PT Programs
A commercial program for external proficiency testing (PT) that has met the criteria established by the
CAP. Analytes within an accepted program are individually evaluated for acceptance.
CAP Staff Inspector
A CAP employee who is a supervisor-eligible or experienced medical technologist (MT) that conducts
inspections on behalf of the CAP.
Checklist
See Accreditation Checklist.
Checklist, Custom
A checklist assigned to an individual laboratory which, based on its activity menu, includes only those
requirements and groups of requirements that apply to the laboratory.
CLIA
An act of Congress—The Clinical Laboratory Improvement Amendments of 1988. The term
CLIA is also used to refer to the regulations that implement the act.
CLIA Number
An identification number assigned to a laboratory by the Centers for Medicare and Medicaid Services.
Clinical Consultant
Individual qualified to consult with and render opinions to the laboratory’s clients concerning the
diagnosis, treatment and management of patient care.
Clinical Laboratory
A facility engaged in the testing of specimens for the diagnosis, management, prevention, and
treatment of disease or the assessment of health. A clinical laboratory usually has one CLIA number,
is located in one building or campus under the leadership of a single laboratory director who is named
on the CLIA certificate, and is owned by one entity.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
118
Clinical Pathology
The major branch of pathology dealing with the identification of disease through chemical
measurement, physical measurement, or culture of bodily fluids and tissues. Clinical pathology
includes, but is not limited to, hematology, urinalysis, chemistry, microbiology, immunology,
transfusion medicine, histocompatibility, related aspects of molecular pathology, and the laboratories
providing service in those areas.
CLIP/CLIP Number
Clinical Laboratory Improvement Program of the US Department of Defense (DOD), an equivalent of
CLIA. The DOD regulates itself with a Memorandum of Agreement with the Department of Health and
Human Services, Centers for Medicare and Medicaid Services due to the unique mission
requirements within the DOD that are not found in the civilian sector. A CLIP identification number is
assigned to the laboratory by CLIP.
CMS
Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration). An
agency within the US Department of Health and Human Services that administers Medicaid,
Medicare, and Child Health Insurance programs and enforces the Clinical Laboratory Improvement
Amendments (CLIA) of 1988 and previous years.
Commissioner, Deputy or Division or State
Individuals responsible for the assignment of inspection team leaders.
Commissioner, Regional
Individuals responsible for overseeing laboratory accreditation activities and recommending
accreditation decisions for a specified set of laboratories.
Consultant
One who provides professional advice or services on request.
Consulting Pathologist
A pathologist who periodically visits a laboratory and serves the role of a technical consultant and/or
performs anatomic pathology services.
Corrective Action
Action taken to eliminate the cause of a detected nonconformity or other undesirable situation.
Council on Accreditation
A CAP council that formulates policy and oversees the accreditation programs. The Chair of the
Council on Accreditation is a member of the CAP Board of Governors.
Credentialing
The process of obtaining, verifying, and assessing the qualifications of a practitioner to provide care in
a health care organization.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
119
Critical PT Performance (Non-regulated analytes)
Failure to attain the minimum satisfactory score for an analyte/test for three consecutive or three of four
consecutive testing events for non-regulated analytes. A laboratory that has repeat critical performance
(four out of five PT events) for a non-regulated analyte/test may be directed to cease testing.
Custom Checklist
See Checklist, Custom.
Deemed Status
The right granted by one organization to a second organization that permits the second organization
to determine whether entities meet requirements imposed by the first organization. For example, the
Centers for Medicare and Medicaid Services has granted deemed status to the CAP, thereby
permitting the CAP to determine whether CAP-accredited laboratories meet the requirements of the
CLIA federal regulations.
Deficiency
Noncompliance with a requirement of the accreditation checklists.
Deficiency Response
For each deficiency cited, the laboratory is required to submit an Inspection Deficiency Response
within 30 calendar days after the inspection. For Phase I deficiencies, the laboratory must submit a
plan of corrective action. For Phase II deficiencies, the laboratory must submit a plan of corrective
action and supporting documentation showing that steps have been taken to correct the deficiency.
De-identification
Removal of information that can be used to identify an individual.
Denial of Accreditation
The decision (by the Accreditation Committee) not to accredit a laboratory based on the findings from
its initial application or CAP inspection.
Director of Laboratory
See Laboratory Director.
Discipline
A CAP-defined term used to describe testing grouped within a major category of clinical laboratory
science (eg, hematology, microbiology, or transfusion medicine).
Distributive Testing
Laboratory testing performed on the same specimen, or aliquot of it, that requires sharing between
two or more laboratories (with different CLIA/CAP numbers) to obtain all data required to complete an
interpretation or calculation necessary to provide a final reportable result for the originally ordered
test.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
120
Doctoral Scientist
An individual who has achieved a doctoral degree in a clinical laboratory discipline such as clinical
chemistry, microbiology, immunology, etc.
Expungement
The elimination of a deficiency from a laboratory’s record when it is determined that the laboratory was
in fact in compliance at the time of the citation.
FDA
1) For laboratories subject to US regulations, FDA refers to the US Food and Drug Administration,
which is the regulatory body under Health and Human Services (HHS) with authority to regulate in
vitro diagnostic products such as kits, reagents, instruments, and test systems. 2) For laboratories not
subject to US regulations, FDA refers to the national, regional, or local authority having jurisdiction
over in vitro diagnostic test systems.
FDA-approved Test
A test that is classified as a Class III medical device and that has been approved by the FDA through
the premarket approval (PMA) process. (21CFR814.3).
FDA-cleared Test
A test that has been cleared by the FDA after analysis of data showing substantial performance
equivalence to other tests being marketed for the same purpose. Such tests typically follow the 510(k)
approval route. (21CFR807).
FDT
See Forensic Drug Testing.
Final List of Deficiencies
A document included in the Accreditation Packet that lists deficiencies (if any) that were found during
a laboratory’s accreditation inspection, exclusive of any deficiencies that were expunged during the
post-inspection process.
Forensic Drug Testing (FDT)
The CAP accreditation program for laboratories that perform drug testing for nonmedical purposes
(eg, workplace drug testing).
General Supervisor
A position defined by the Clinical Laboratory Improvement Amendments (CLIA) of 1988 as the
individual who provides day-to-day supervision of testing personnel and reporting of testing results in
a laboratory that performs high-complexity testing.
High Complexity
Rating given by the FDA to commercially marketed in vitro diagnostic tests based on their risks to public
health. Tests in this category are seen to have the highest risks to public health.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
121
Immediate Review Criteria (IRC)
Findings that indicate that review of a laboratory’s inspection results should be given a higher priority
throughout the accreditation review process. Such findings include an excessive percentage of
deficiencies and problems with proficiency testing.
Inspection Instance (II)
A numerical identifier for each inspection that groups together laboratories and sections\departments
(usually a single campus or geographic area).
Inspection Team Leader
The individual responsible for assembling and leading a team of inspectors and for submitting the
Inspector’s Summation Report to the CAP.
Inspection Team Member
An individual designated by the inspection team leader to perform a specific aspect of the inspection.
Inspection Unit (IU)
One or more laboratories that are inspected at the same time by an inspection team. An IU is used to
track that the laboratories in the IU have fulfilled their inspection obligation.
Inspector
An experienced pathologist, resident or fellow in pathology, clinical scientist, medical technologist, or
other laboratory personnel, as appropriate, who acts as an inspection team member or team leader.
Inspector’s Inspection Packet
The packet of materials sent to an inspection team leader to be used to conduct an inspection.
Included are the appropriate checklists, laboratory synopsis reports, the Laboratory Accreditation
Manual, previous inspection results, and Inspector’s Summation Report forms, and other needed
materials.
Inspector’s Summation Report (ISR)
The form returned by the inspection team leader documenting inspection deficiencies,
recommendations, and inspector’s comments.
IRC Laboratory
See Immediate Review Criteria.
Laboratory
Term used to refer to a clinical laboratory, biorepository, forensic drug testing laboratory, or reproductive
laboratory participating in the CAP accreditation programs.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
122
Laboratory Developed Test (LDT)
For the purposes of interpreting the checklist requirements, a laboratory-developed test (LDT) is
defined as follows: A test used in patient management that has both of the following features:
1. The test is performed by the clinical laboratory in which the test was developed wholly or
in part; AND
2. The test is neither FDA-cleared nor FDA-approved.
Laboratory Director
The individual who is responsible for the overall operation and administration of the laboratory,
including provision of timely, reliable and clinically relevant test results and compliance with
applicable regulations and accreditation requirements. This individual is listed on the laboratory's
CAP and CLIA certificates (as applicable).
Laboratory Inspection Packet
A packet of information sent to the laboratory prior to the inspection that contains the laboratory -
specific activity menu, checklists, deficiency response sheets, and instructions on how and when to
respond to deficiencies.
Laboratory-specific Activity Menu
See Accreditation Unit Activity Menu.
License
Right or permission granted in accordance with the law by a competent authority to engage in some
business or occupation, which, but for such license, would be unlawful. For laboratories, a license may
be granted by a municipal, state, or federal authority. For physicians, in the United States, a license is
granted by the State Board of Medical Examiners.
List of Deficiencies
The set of checklist requirements that were established as deficiencies at an inspection of a specific
laboratory.
Master Activity Menu
See Activity Menu, Master.
Moderate Complexity
Rating given by the FDA to commercially marketed in vitro diagnostic tests based on their risks to
public health.
Non-routine Inspection
Any inspection performed on-site in addition to the biennial routine inspection. Non-routine inspections
may be performed for a variety of reasons, including (without limitation) a change of laboratory
director, addition of disciplines, determination of whether the laboratory has met conditions imposed
by the CAP, or investigation of a complaint.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
123
Nonwaived
Tests categorized as either moderately complex (including provider-performed microscopy) or high
complexity according to a scoring system used by the FDA.
Pathologist
A physician who has successfully completed an approved graduate medical education program in
pathology.
Pathologist Assistant
An individual qualified to perform high-complexity testing (under CLIA regulations), with appropriate
training and/or education, who assists the pathologist in gross examination of surgical specimens,
autopsies, and other procedures.
Pathology
The specialty of the practice of medicine dealing with the causes and nature of disease, including
diagnosis, prognosis, and response to treatment, generally involving examination of biologic materials
(eg, tissue, blood, or other fluids).
Personnel
The collective group of employees and contractors employed in the laboratory organization.
Contractors may include those individuals contracted by the laboratory, such as pathologists, medical
technologist, or nurses who perform patient testing. It would not include those individuals contracted
outside the authority of the laboratory, such as medical waste disposal contractors, instrument service
representatives, or cleaning contractors.
Point-of-Care Testing
Testing that is performed at or near the site where the patient is located, that does not require
permanent dedicated space, and that is performed outside the physical facilities of the clinical
laboratories.
Policy
1) Set of basic principles or guidelines that direct or restrict the facility's plans, actions, and
decisions; 2) Statement that tells what should or should not be done.
Postanalytic Phase (post-examination process)
Processes following the analysis (examination) of patient specimens, including review, formatting,
interpretation, verification, reporting and transmission of the results, and storage of samples and
results.
Preanalytic Phase (pre-examination process)
Processes prior to the analytic examination of patient specimens, including, in chronological order: the
clinician’s request, test order, preparation of the patient, collection of the primary sample,
transportation to and within the laboratory, and sample preparation.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
124
Preliminary Accreditation
Accreditation status that is applied to a laboratory when there is an urgent need for an accreditation
decision prior to completion of the usual course of action for an accreditation decision, or when
accreditation is required prior to the commencement of patient testing. This status remains in effect
until such time the final accreditation process has taken its course and a final accreditation decision is
made.
Preventive Action
Action taken to eliminate the cause of a potential nonconformity or any other undesirable potential
situation.
Primary Source Verification Report
A document, usually prepared by a third party agent or company, that confirms that a job applicant's
degree, certificate, or diploma is authentic, licenses were granted, and reported work history
(company names, locations, dates and positions held) is accurate. The confirmation is obtained
through direct contact with an institution, former employer, or their authorized agents.
Probation
An accreditation status assigned by the Accreditation Committee if any of the following inspection
findings exist:
• Documentation is insufficient to determine compliance with the CAP’s standards within the
Standards for Laboratory Accreditation.
• The committee wishes to monitor the laboratory’s progress in correcting deficiencies.
• The laboratory has engaged in conduct contrary to the policies of the CAP but such
conduct is not sufficient to warrant denial or revocation of accreditation.
A laboratory on probation may continue to provide testing as an accredited laboratory.
Probation with Immediate Jeopardy
A status assigned by the Accreditation Committee when noncompliance with one or more
requirements of the CAP has already caused, is causing, or is likely to cause serious injury, harm, or
death to individuals served by the laboratory and/or to the health or safety of the general public and/or
to laboratory workers or visitors.
Procedure
1) Specified way to carry out an activity of a process (also referred to by ISO as "work instructions");
2) Set of steps performed that tells "how to do it" to achieve a specified outcome, including decisions
to be made.
Process
1) Set of interrelated or interacting activities that transforms inputs into outputs; 2) Series of events,
stages, or phases that takes place over time that tells "what happens" or "how it works."

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
125
Proficiency Testing (PT) (Also termed: External Quality Assessment [EQA])
Evaluation of participant (or individual) performance against pre-established criteria by means of
interlaboratory comparisons.
Proficiency Testing Performance <100% Report
A report included in the Inspector Inspection Packet that shows all variant PT performances (any
score that is less than 100%) for the last six PT mailing events for the laboratory. This report is
intended to help the inspector focus on possible problem areas. All variant PT results must be
investigated, and corrective action documented.
Provider Performed Microscopy (PPM)
Testing that is personally performed by a physician in conjunction with the physical examination or
treatment of a patient. PPM tests are limited to those listed in the accreditation checklists.
Quality Control
An integral component of quality management composed of the aggregate of processes and
techniques used to detect, reduce, and correct deficiencies in an analytical process. Quality control
(QC) is a surveillance process in which the actions of people and performance of equipment and
materials are observed in some systematic, periodic way that provides a record of consistency of
performance and of action taken when performance does not conform to standards set by the
laboratory. QC is a set of procedures designed to monitor the test method and the results to ensure
test system performance; QC includes testing control materials, charting the results and analyzing
them to identify sources of error, and determining, performing, and documenting any remedial action
taken as a result of this analysis.
Quality Improvement
A systematic method used to identify opportunities for improvement in clinical and nonclinical systems.
Quality Management
All activities of the overall management function that determine quality policy objectives and
responsibilities and the implementation of them, including the preanalytic, analytic, and postanalytic
phases of testing.
Quality Management System
Set of processes, policies, procedures, and resources designed to ensure high quality in an
organization’s services.
Referral Laboratory
The laboratory that receives a specimen for analysis from another laboratory.
Referring Laboratory
The laboratory that initiates the transfer of a specimen to another testing facility for analysis.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
126
Repeat Unsuccessful Proficiency Testing (PT) Performance (Cease Testing for Regulated
Analytes)
Unsatisfactory PT performance for a CLIA-regulated analyte/test/subspecialty in three consecutive or
two sets of “two out of three” (a failure in one event may be included in more than one set) over
twelve PT events. A laboratory that has repeat unsuccessful PT performance for a regulated
analyte/test/subspecialty may be directed to cease testing for six months.
Reproductive Laboratory Accreditation Program (RLAP)
The CAP accreditation program that accredits laboratories that perform andrology and embryology
testing.
Required Analyte
An activity for which the CAP Accreditation Program requires PT enrollment and participation in a
CAP-accepted PT Program. Both waived and nonwaived activities are included in the list of required
analytes.
Responsibility
A duty or task that an individual is required or expected to do.
Reviewing Commissioner
The commissioner (ordinarily a regional commissioner) who reviews the Inspector’s Summation
Report and the laboratory’s responses and makes an accreditation recommendation to the
Accreditation Committee.
Revocation of Accreditation
Termination of a laboratory’s existing accreditation by the Accreditation Committee.
RLAP
See Reproductive Laboratory Accreditation Program.
Root Cause Analysis (RCA)
A systematic process for identifying the causal factor(s) that underlie errors or potential errors in care.
Section Director
The individual who is responsible for the technical and/or scientific oversight of a specialty or section
of the laboratory.
Section Unit (SU)
An operational area or department of a laboratory, which may correspond to a laboratory specialty
(eg, hematology, chemistry).
Self-Inspection
The laboratory-performed inspection that occurs in the year between CAP-performed inspections.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
127
Standards
Collective term used to describe the Standards for Laboratory Accreditation, Standards for Forensic
Drug Testing Accreditation, Standards for Reproductive Laboratory Accreditation, and Standards for
Biorepository Accreditation as published by the CAP Council on Accreditation. The Standards are the
core principles of the CAP’s accreditation programs.
SU
See Section Unit.
Subdiscipline
A CAP-defined term used to describe related testing activities that reside under a particular discipline
(eg, parasitology, virology, mycology).
Subject to US Regulations
Laboratories located within the United States, and laboratories located outside of the US that have
obtained or applied for a CLIA certificate to perform laboratory testing on specimens collected in the
US and its territories for the assessment of the health of human beings.
Supervisor
A person responsible for the daily activities of a section unit.
Suspension
Removal of accreditation from one or more sections of a laboratory. The suspended sections(s) may
not provide testing as an accredited laboratory. This status is assigned by the Accreditation
Committee pending the laboratory meeting conditions assigned by the committee. The suspended
status may exist for no more than 45 days.
Target Inspection Date
The date that signifies the end of the calendar day window during which the inspection should occur.
For accredited laboratories, the target inspection date and the anniversary date are usually the same.
Technical Consultant
A position defined by CLIA as the individual responsible for the technical and scientific oversight of a
laboratory performing moderately complex testing. The technical consultant may or may not be the
same individual as the laboratory director, depending on the qualifications of the laboratory director
and the manner in which the laboratory is organized. The technical consultant may be a pathologist,
other physician, doctoral scientist, or possess other required qualifications.
Technical Supervisor
A position defined by CLIA as the individual responsible for technical and scientific oversight of a
laboratory performing high complexity testing. The qualifications required for the technical supervisor
may vary, depending on the laboratory specialty. The technical supervisor may be a pathologist, other
physician, doctoral scientist, or possess other required qualifications.

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
128
Telepathology
The practice of pathology and cytology in which digitized or analog video still image(s), or other data
files are examined and an interpretation is rendered that is included in a formal diagnostic report in the
patient record. It also includes the review of images by a cytotechnologist when a judgement of
adequacy is recorded in the patient record.
Termination of Accreditation
The process by which a laboratory’s accreditation is ended and all regulatory agencies involved with
the laboratory are notified. Reasons for termination include:
• Denial of a laboratory’s accreditation after an inspection.
• Initiation of termination by the laboratory itself when it no longer wishes to participate in the
CAP’s laboratory accreditation programs. The laboratory is responsible for notifying CAP staff
of its intention to discontinue coverage.
• Failure to return reapplication materials within a specified time frame. The termination
will occur after reminder options have been exhausted. Letters will be sent to the laboratory and
the regional commissioner stating that the laboratory has been terminated because completed
reapplication materials were not returned to CAP.
• Merger of two or more laboratories, which results in the accreditation of a single laboratory.
The laboratories that are no longer effective (eg, discontinued CLIA number) will be
terminated, and the surviving laboratory’s record will be updated to reflect all changes due
to the merger.
• Failure to meet the standards set forth in the CAP’s accreditation Standards.
Terms of Accreditation
Administrative obligations of a CAP-accredited laboratory.
Test
A qualitative, semiqualitative, quantitative, or semiquantitative procedure for detecting the presence of,
or measuring of an analyte.
Test Complexity
Test categorization, as defined by CLIA (42CFR493.17). Tests are divided into waived, moderately
complex, and highly complex categories, based on the scientific and technical knowledge, training
and experience, and interpretation and judgment required to perform the test; and the degree of
difficulty in the handling of reagents and materials, operational steps, calibration, and maintenance.
Testing Personnel
Individuals responsible for performing laboratory assays and reporting laboratory results.
Unsatisfactory Proficiency Testing (PT) Performance
Failure to attain at least 80% for a regulated analyte/subspecialty/specialty (ABO, Rh, and
Compatibility Testing requires 100%) for a testing event. Clerical errors or data omissions are
considered PT failures. For nonregulated analytes, satisfactory performance will vary based on the
number of challenges. (Refer to the CAP’s PT/External Quality Assurance Toolbox available through
e-LAB Solutions Suite for more information.)

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
129
Unsuccessful Proficiency Testing (PT) Performance
Failure to attain at least 80% for a regulated analyte/subspecialty/specialty for 2 consecutive or 2 out
of 3 testing events. (ABO, Rh, and Compatibility testing requires 100%) Unsuccessful PT
performance and unsuccessful PT participation are synonymous. For nonregulated analytes,
satisfactory performance will vary based on the number of challenges. Refer to the CAP’s
PT/External Quality Assurance Toolbox available through e-LAB Solutions Suite for more
information.
Virtual Inspection
CAP model for remotely assessing laboratory compliance that utilizes video conferencing, digital file
transfer, and live streaming to perform an interactive review of laboratory policies and procedures,
records, and processes.
Visitor
An individual entering the laboratory who is not considered personnel.
Volunteer Inspector
A person who conducts inspections for the CAP’s laboratory accreditation programs without
monetary compensation. All labs enrolled in the CAP’s laboratory accreditation programs are
expected to provide a volunteer inspector team once every two years to conduct an inspection of
another similar lab, if asked.
Waived
A category of tests defined as “simple laboratory examinations and procedures which have an
insignificant risk of an erroneous result.” Laboratories performing waived tests are subject to minimal
regulatory requirements.
For laboratories subject to US regulations, these tests are assigned to the waived category by the US
Food and Drug Administration (FDA).

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
130
Appendix E:
CAP Accreditation Program Website Tools
The table includes a list of resources available on cap.org
. The fastest way to locate these resources is
to use the search option using the path indicated in the Location column below. Resources marked
with an asterisk (*) require e-LAB Solutions Suite log in access. The link to access e-LAB Solutions
Suite is on the CAP Home Page.
Tool
Location
Description
Accreditation Checklist
Download *
Log into e-LAB Solutions Suite:
Search – Accreditation
Checklists
Online tool to download checklists,
with options to select:
• Checklist module
• Checklist edition
• Checklist Type:
o Master (displays all
requirements with
references and
inspector
instructions)
o Custom (displays
applicable requirements
based on lab test menu)
o Changes only (displays
changes from previous to
current edition)
• Checklist format (PDF, Word/XML,
or Excel)
Accredited Laboratory
and Biorepository
Directory
CAP Home:
Go to: Laboratory Improvement
– Accreditation – Find a CAP
Accredited Laboratory
Searchable database of CAP-
accredited laboratories or
biorepositories
Biorepository
Resources*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Quality Management
Quality management resources for
biorepositories

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
131
CAP Accreditation
Programs Policy
Manual*
Log into e-LAB Solutions Suite:
Search – CAP Accreditation
Programs Policy Manual -OR-
Go to: Accreditation Resources
– Accreditation
Manuals/Retention Guidelines
– CAP Accreditation Programs
Policy Manual
CAP Accreditation Program
administrative policies approved by
the CAP’s Council on Accreditation
CAP Accreditation
Standards*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Accreditation
Manuals/Retention Guidelines
The principles of the CAP
accreditation programs:
• Laboratory Accreditation
Program (LAP)
• Forensic Drug Testing
Program (FDT)
• Reproductive Laboratory
Accreditation Program
(RLAP)
• Biorepository Accreditation
Program (BAP)
CAP Cancer
Protocols Templates
CAP Home: Search - Cancer
Protocols
Guidelines for collecting the essential
data elements for complete reporting
of malignant tumors and optimal
patient care
CAP Evidence-
Based Guidelines
CAP Home: Search – CAP
Guidelines
Evidence-based guidelines and
consensus recommendations
developed by the CAP Pathology and
Laboratory Quality Center, along with
its professional partners, intended to
improve diagnostic and clinical
decision making
CAP Personnel
Requirements by
Test Complexity*
Log into e-LAB Solutions Suite:
Search – CAP Personnel
Requirements by Test
Complexity
Listing of CAP personnel
qualifications for all CLIA-defined
roles defined based on the
complexity of tests performed

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
132
Checklist Errata
Log into e-LAB Solutions Suite:
Search – Checklist Errata
Listing of errors or changes in
checklist interpretation in a checklist
edition after publication (not all
editions have errata)
Checklist
Requirement
Questions &
Answers*
Log into e-LAB Solutions Suite:
Search – Checklist
Requirement Q & A -OR-
Go to: Accreditation Resources
– Checklist Requirement Q&A
• Links to commonly asked
accreditation questions on a
variety of topics
CLIA Director
Education,
Information and
Resources*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– CAP Laboratory Director
Education, Information &
Resources
Tools to educate laboratory directors
on director responsibilities
COVID Checklist
Requirements*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Inspector Training and Tools
– COVID-19 Checklist
Requirements Tip Sheet
Tip sheet for inspection laboratories
performing COVID-19 testing
Deficiency Response
Instructions and
Forms*
Log into e-LAB Solutions Suite:
Search – Self and Post
Inspection Toolbox
Instructions and forms for responding
to CAP inspection deficiencies
eAlerts
CAP Home: Search: - eAlerts
-OR- Go to: Laboratory
Improvement – News and
Updates
Links to important notifications sent
to laboratories about changes to the
CAP’s accreditation programs
Fast Focus on
Compliance
CAP Home: Search – Fast
Focus on Compliance -OR-
Go to: Laboratory Improvement
– Accreditation – Educational
Resources
Mini-training vignettes for inspectors
that use real world examples to
Provide practical approaches to
handle new and perplexing topics
Focus on
Compliance
Webinars – Library of
Past Webinars*
Log into e-LAB Solutions Suite:
Search– Focus on
Compliance -OR-
Go to: Accreditation Resources
– Focus on Compliance
Archived webinar materials including:
• Presentations
• Toolkits
• Questions & Answers

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
133
Focus on
Compliance
Webinars -
Registration
CAP Home: Search – Focus
on Compliance -OR-
Go to: Laboratory
Improvement – Accreditation –
Educational Resources –
Focus on Compliance
Webinars
Registration information for the
complimentary series of accreditation
educational webinars
• Designed for laboratory
directors, managers, and
technologists
•
Provides expert knowledge
and regulatory compliance
insight
Guide to CAP Accreditation
for International Laboratories
CAP Home: Search – Focus
on Compliance -OR-
Go to: Laboratory
Improvement – International
Laboratories
Important information for
international laboratories to
consider when applying for CAP
accreditation
Individualized Quality Control
Plan (IQCP) Toolbox*
Log into e-LAB Solutions Suite:
Search – Focus on
Compliance -OR-
Go to: Accreditation Resources
– IQCP Toolbox
Web page includes the following
tools:
• IQCP frequently asked
questions
• IQCP Eligibility Flow Chart
• IQCP List form and
• instructions
• IQCP Quality Assessment –
example form
Jointly developed microbiology
tools for antimicrobial susceptibility
testing, identification systems, and
media
•
Inspector tip sheet
•
IQCP Webinar
Inspecting a Biorepository*
Log into e-LAB Solutions Suite:
Go to: BAP Laboratory
Inspection
Inspection guides for BAP:
• Team leaders
• Team members
• Inspection Document
Review
Inspection Resources –
including Virtual and
Modified Inspections*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Inspector Training and Tools
Tools for performing virtual
inspection and understanding the
modified inspection process

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
134
Inspection Summation
Report
(ISR)*
Log into e-LAB Solutions Suite:
Search – Inspection
Summation Report
Web page that allows laboratories
to generate reports with deficiency
information from current and
previous CAP inspections
Inspector Guides and Tip
Sheets*
Log into e-LAB Solutions Suite:
Search – Inspector Training
and Tools -OR-
Go to: Accreditation Resources
– Inspector Training and Tools
Team leader and team member
guides
Inspector Training Modules
CAP Home: Search –
Inspector Training -OR-
Go to: Laboratory
Improvement - Accreditation
Online Inspector Training modules
for:
• Team leaders
• Team members
Laboratory Accreditation
Forms*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Self & Post Inspection
Toolbox
Forms for download include:
• Inspection Preparation
Checklist
• Instructions for Deficiency
Response
• Deficiency Response Form
• Self-inspection tips
• Deficiency Response
Signature Form
• Self-inspection Verification
Form
• Self-inspection Deficiency
Summary Form
Laboratory Accreditation
Manual
*
Log into e-LAB Solutions
Suite: Search– Laboratory
Accreditation Manual
-
OR- Go to: Accreditation
Resources
– Accreditation
Manuals/Retention Guidelines
Resource for laboratories and
inspectors on the inspection and
accreditation processes

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
135
Laboratory Inspection
Preparation Course*
Log into e-LAB Solutions Suite:
Search – Laboratory
Inspection Preparation -OR-
Go to: Accreditation Resources
– Laboratory Inspection
Preparation Course
Short videos on a variety of
accreditation topics designed to
help prepare for CAP inspection
Navigating the Virtual
Inspection*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Laboratory Inspection
Preparation Course
Tips on using the SharePoint
software tool to share and review
documents online during virtual
inspections
Organization Profile*
Log into e-LAB Solutions Suite:
Go to: Organizational Profile
Tool to view and update
laboratory data such as:
• Demographics
• General information on
laboratory operation
• Regulatory relationships (eg,
CLIA number, state
licensure)
• Personnel roles
Laboratory sections/departments
and activities performed in each
Performance
Analytics Dashboard*
Log into e-LAB Solutions Suite:
Search – Dashboard
Web-based reporting solution,
free to CAP-accredited
laboratories, that is updated daily
to:
• Provide a comprehensive
data view for proficiency
testing and accreditation
performance
• Allow laboratories to
benchmark individual
performance against peers
and CAP-wide performance

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
136
Proficiency Testing
(PT)/External Quality
Assurance Toolbox *
Log into e-LAB Solutions Suite:
Search – PT Toolbox
PT resources including:
• PT compliance frequently asked
questions
• PT troubleshooting guides
• PT result failure
investigation forms
• Alternative performance
assessment guide
Proficiency Testing
Compliance Notice (PTCN)*
Log into e-LAB Solutions Suite:
Search – PT Compliance
Notice -OR- Go to:
Accreditation Resources –
Proficiency Testing
(PT)/External Quality
Assurance (EQA) Toolbox
Instructions for actions to take if
a PT compliance notice is
received for:
• Missing PT enrollment
• Non-participation in PT
• Unacceptable PT
performance
PT/EQA failure types and codes
Quality Management (QM)*
Log into e-LAB Solutions Suite:
Go to: Accreditation Resources
– Quality Management
Quality management tools for
establishing and monitoring
quality indicators and educational
content on quality management
systems and root cause analysis
Retention of Laboratory
Records
and Materials *
Log into e-LAB Solutions Suite:
Search – Retention of
Laboratory Records and
Materials -OR- Go to:
Accreditation Resources –
Accreditation
Manuals/Retention Guidelines
CAP guidelines for the retention
of laboratory records and
materials

College of American Pathologists
Laboratory Accreditation Manual 2022 Edition
137
Root Cause Analysis Toolkit*
Log into e-LAB Solutions Suite:
Search – Root Cause
Analysis -OR- Go to:
Accreditation Resources –
Quality Management
Spreadsheet for performing a
root cause analysis that helps to:
• Define a problem
• Map current processes
• Find the root cause
• Develop a solution
• Implement a solution
• Assess effectiveness
RCA completed example and
check sheet
Self-Inspection Tools*
Log into e-LAB Solutions Suite:
Search – Self and Post
Inspection Toolbox
Tips for performing a self-
inspection and self-inspection
forms
CAP accreditation: Share the good news!
It’s time to let everyone know about your team’s hard work. CAP accreditation means your laboratory has met
the highest standards for patient care, and that’s a distinction well worth sharing. The CAP Communications
team will help you spread the news with:
• A news release and newsletter article template: Ask for the templates by sending an email to
[email protected]. Then you will simply fill in the important information and send the completed templates
to your Communications, Public Relations/Media, and or Marketing departments for help with
distribution.
o The news release can be distributed externally to your community newspapers or a newswire
service for a wider, more national reach.
o The newsletter article can be placed in all your internal publications.
o The CAP can also provide news release templates in the following languages:
Simplified Chinese
Spanish (Central/South America)
Portuguese (Brazil)
Arabic
Japanese
Korean
German
• Suggested social media language and CAP quotes: Publicize your accreditation via your institution’s
Facebook, Twitter, or other social media channels. We have social media language and suggested
quotes of support from the CAP Council on Accreditation chair to use in your news release and
newsletter article. Ask for social media language by sending an email to medi[email protected].
• A photo of your laboratory team: A picture is worth a thousand words! Just snap a photo of the
laboratory team and invite your CEO to be a part of the picture, too. The photo can accompany your
news release, newsletter article, or both!
o The CAP would also like the photo, so we can let others know about your achievement! Please
send the photo, along with the names of your laboratory director and CEO (if applicable) to:
o Once a month, we will feature a CAP-accredited laboratory and photo on
cap.org.
• Download the CAP accreditation mark: The accreditation mark positions laboratory professionals as
an integral part of the health care community and the patient care team. To download the accreditation
mark, along with the usage guidelines, please visit e-LAB Solutions Suite (login required).
Questions? Send us an email at media@cap.org

Let Them Know You’ve Earned the Mark
The CAP certication mark recognizes your organization for achieving CAP accreditation, something you share
with almost 8,000 laboratories worldwide. The mark is a way to display to peers, patients, and the public
that you’ve attained CAP accreditation through the most respected and recognized laboratory accreditation
program in the world.
Proudly display your CAP certication mark on your website, advertisements, laboratory reports, and in your
patient areas to communicate your CAP accreditation status. We know you’re proud, and we’re proud, too.
To access and download your CAP certication mark, please log in to your e-LAB Solutions™ account,
or contact the CAP Customer Contact Center.
College of American Pathologists
325 Waukegan Road
Northeld, IL 60093-2750
800-323-4040
847-832-7000
© 2022 College of American Pathologists. All rights reserved. 29957.0922
cap.org
29957_LAM_CVR_Bk2_.indd 229957_LAM_CVR_Bk2_.indd 2 2/22/22 10:52 AM2/22/22 10:52 AM
