
Chemistry Guide
DNA Sequencing by Capillary Electrophoresis
Applied Biosystems Chemistry Guide | Second Edition

Chemistry Guide
DNA Sequencing by Capillary Electrophoresis
Applied Biosystems Chemistry Guide
Second Edition
For Research Use Only. Not for use in diagnostic procedures.
Information in this document is subject to change without notice.
APPLIED BIOSYSTEMS DISCLAIMS ALL WARRANTIES WITH RESPECT TO THIS DOCUMENT, EXPRESSED OR IMPLIED, INCLUDING
BUT NOT LIMITED TO THOSE OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. TO THE FULLEST EXTENT
ALLOWED BY LAW, IN NO EVENT SHALL APPLIED BIOSYSTEMS BE LIABLE, WHETHER IN CONTRACT, TORT, WARRANTY, OR UNDER
ANY STATUTE OR ON ANY OTHER BASIS FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL
DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING BUT NOT LIMITED TO THE USE THEREOF,
WHETHER OR NOT FORESEEABLE AND WHETHER OR NOT APPLIED BIOSYSTEMS IS ADVISED OF THE POSSIBILITY OF SUCH
DAMAGES.
NOTICE TO PURCHASER:
PLEASE REFER TO THE RESPECTIVE INSTRUMENT'S USER GUIDES, PRODUCT INSERTS, OR APPLIED BIOSYSTEMS PRODUCT PAGE
FOR LIMITED LABEL LICENSE OR DISCLAIMER INFORMATION.
TRADEMARKS:
Applied Biosystems, AB (Design), ABI PRISM, BigDye, BloodPrep, GeneAmp, MicroAmp, MicroSeq, NucPrep, Primer Express, SeqScape, SNaPshot,
VariantSEQr, and Veriti are registered trademarks and Hi-Di, KB, methylSEQr, POP, POP-4, POP-5, POP-6, POP-7, SNPbrowser, TargetSeq, Variant
Reporter, and XTerminator are trademarks of Applied Biosystems or its affiliates in the US and/or certain other countries.
AmpliTaq, AmpliTaq Gold, and TaqMan are registered trademarks of Roche Molecular Systems, Inc.
PicoGreen is a registered trademark of Molecular Probes, Inc.
All other trademarks are the sole property of their respective owners.
© Copyright 2009, Applied Biosystems. All rights reserved.
Part Number 4305080 Rev. C
05/2009

Contents
DNA Sequencing by Capillary Electrophoresis Chemistry Guide iii
Preface
How to Use This Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . vii
How to Obtain Support . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . viii
Safety and EMC Compliance Information
Safety Conventions Used in This Document . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . x
Chemical Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi
Chemical Waste Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi
Biological Hazard Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi
Chapter 1 Introduction to DNA Sequencing
DNA Sequencing Basics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Cycle Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
Capillary Electrophoresis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
Data Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Automated DNA Sequencing Workflow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
Chapter 2 Applications Overview
DNA Sequencing Applications and Approaches . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
De Novo Sequencing of Genomes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Resequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20
Epigenetics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23
Microbial Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Chapter 3 DNA Template Preparation
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
Preparing Vector-Based DNA Templates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33
Preparing Genomic DNA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34
Preparing PCR DNA Templates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
Primer Design and Quantitation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
Purifying PCR Products for Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41

iv DNA Sequencing by Capillary Electrophoresis Chemistry Guide
DNA Template Quality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44
DNA Template Quantity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45
Preparing Templates for Bisulfite Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46
Chapter 4 Cycle Sequencing
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54
Choosing a Sequencing Chemistry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 55
Reagent and Equipment Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61
DNA Quantity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 63
Using DNA Template Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64
Using BigDye Terminators and dGTP BigDye Terminators . . . . . . . . . . . . . . . . . . . . . . . 66
Using dRhodamine Terminators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72
Using BigDye Primers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73
Direct Sequencing From Cultures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75
Bisulfite Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 77
Chapter 5 Purification of Extension Products
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 86
Purification with the BigDye
®
XTerminator
™
Purification Kit . . . . . . . . . . . . . . . . . . . . . . 88
Purification by Ethanol Precipitation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 96
Purification with Spin Columns and Spin Plates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 116
Alternative Purification Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119
Sample Preparation for Electrophoresis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 120
Chapter 6 Capillary Electrophoresis
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128
Calibrating the Instrument . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131
Creating the Plate Record in Data Collection Software . . . . . . . . . . . . . . . . . . . . . . . . . 136
Defining the Results Group . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 136
Defining the Instrument Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 137
Defining the Analysis Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 143
Run Parameters for Instrument and Analysis Protocols . . . . . . . . . . . . . . . . . . . . . . . . 151
Chapter 7 Data Analysis
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 158
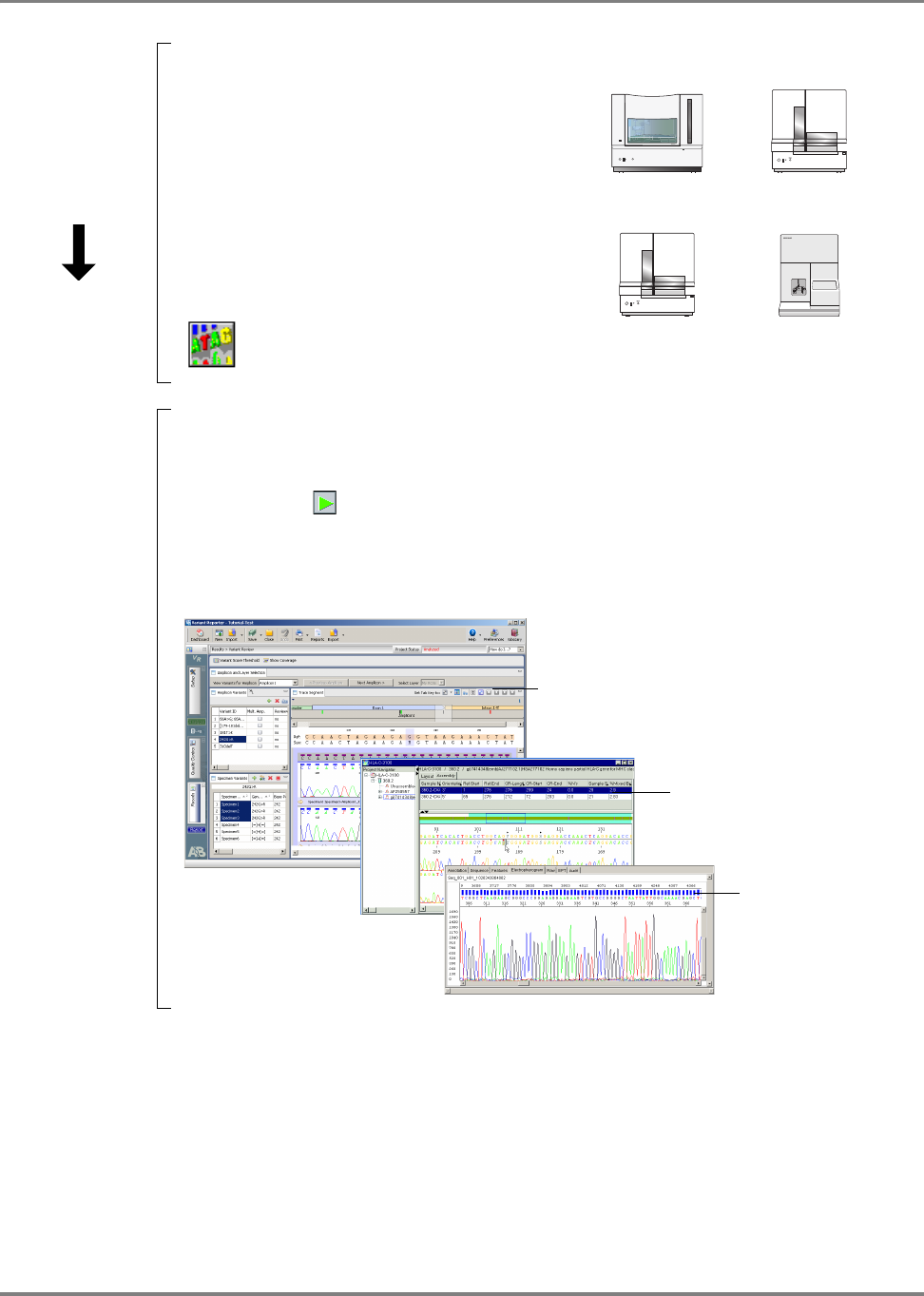
Reviewing Data with Sequence Scanner Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164
Analyzing Data with Sequencing Analysis Software . . . . . . . . . . . . . . . . . . . . . . . . . . . 170
Analyzing Data with Variant Reporter
™
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 175

DNA Sequencing by Capillary Electrophoresis Chemistry Guide v
Analyzing Data with SeqScape
®
Software . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 181
Analyzing Data with MicroSeq
®
ID Analysis Software . . . . . . . . . . . . . . . . . . . . . . . . . 188
Chapter 8 Troubleshooting
Troubleshooting Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 194
Troubleshooting Workflow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 194
Table of Troubleshooting Symptoms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 201
Troubleshooting Examples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 203
Troubleshooting Index . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 255
Appendix A Product Information
Peak Color/Base Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 262
Control Sequences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 263
Appendix B Parts List
Kits and Reagents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 266
Instrument Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 270
Software Products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 278
Miscellaneous Documents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 279
Bibliography
Glossary
Index

vi DNA Sequencing by Capillary Electrophoresis Chemistry Guide

viiDNA Sequencing by Capillary Electrophoresis Chemistry Guide
Preface
How to Use This Guide
Purpose of This
Guide
This chemistry guide is designed to familiarize you with Applied Biosystems genetic
analyzers for automated DNA sequencing by capillary electrophoresis, to provide
useful tips for ensuring that you obtain high quality data, and to help troubleshoot
common problems.
Chapter 4, “Cycle Sequencing,” Chapter 5, “Purification of Extension Products,” and
Chapter 8, "Troubleshooting" are each bound separately so they can be removed from
the binder for easy use in the laboratory. Chapter 4 and Chapter 5 contain many
protocols used in DNA sequencing.
Audience
This guide is intended for novice and experienced users who perform automated
DNA sequencing.
Assumptions
This guide assumes that your Applied Biosystems genetic analyzer has been installed
by an Applied Biosystems technical representative.
This guide also assumes that you have a working knowledge of the Windows
operating system.
Te xt Co n v e n t i o n s
This guide uses the following conventions:
• Bold text indicates user action. For example:
Ty pe 0, then press Enter for each of the remaining fields.
• Italic text indicates new or important words and is also used for emphasis.
For example:
Before analyzing, always prepare fresh matrix.
• A right arrow symbol () separates successive commands you select from a
drop-down or shortcut menu. For example:
Select FileOpenSpot Set.
Right-click the sample row, then select View Filter View All Runs.
User Attention
Words
Two user attention words appear in Applied Biosystems user documentation. Each
word implies a particular level of observation or action as described below:
Note: – Provides information that may be of interest or help but is not critical to the
use of the product.
IMPORTANT! – Provides information that is necessary for proper instrument
operation, accurate chemistry kit use, or safe use of a chemical.

viii DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Preface
Examples of the user attention words appear below:
Note: The Calibrate function is also available in the Control Console.
IMPORTANT! To verify your client connection to the database, you need a valid user
ID and password.
Safety Alert
Words
Safety alert words also appear in user documentation. For more information, see
“Safety Alert Words” on page x.
How to Obtain Support
For the latest services and support information for all locations, go to
www.appliedbiosystems.com, then click the link for Support.
At the Support page, you can:
• Search through frequently asked questions (FAQs)
• Submit a question directly to Technical Support
• Order Applied Biosystems user documents, MSDSs, certificates of analysis,
and other related documents
• Download PDF documents
• Obtain information about customer training
• Download software updates and patches
In addition, the Support page provides access to worldwide telephone and fax
numbers to contact Applied Biosystems Technical Support and Sales facilities.

ixDNA Sequencing by Capillary Electrophoresis Chemistry Guide
Safety and EMC Compliance Information
This section covers:
Safety Conventions Used in This Document. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .x
Chemical Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi
Chemical Waste Safety . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi
Biological Hazard Safety. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . xi

x DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Safety and EMC Compliance Information
Safety Conventions Used in This Document
Safety Alert
Words
Four safety alert words appear in Applied Biosystems user documentation at points
in the document where you need to be aware of relevant hazards. Each alert
word—IMPORTANT, CAUTION, WARNING, DANGER—implies a particular
level of observation or action, as defined below.
Definitions
IMPORTANT!
– Indicates information that is necessary for proper instrument
operation, accurate chemistry kit use, or safe use of a chemical.
– Indicates a potentially hazardous situation that, if not avoided,
may result in minor or moderate injury. It may also be used to alert against unsafe
practices.
– Indicates a potentially hazardous situation that, if not avoided,
could result in death or serious injury.
– Indicates an imminently hazardous situation that, if not avoided,
will result in death or serious injury. This signal word is to be limited to the most
extreme situations.
Except for IMPORTANTs, each safety alert word in an Applied Biosystems
document appears with an open triangle figure that contains a hazard symbol. These
hazard symbols are identical to the hazard symbols that are affixed to Applied
Biosystems instruments.
Examples
The following examples show the use of safety alert words:
IMPORTANT! You must create a separate sample entry spreadsheet for each 96-well
plate.
The lamp is extremely hot. Do not touch the lamp until it has
cooled to room temperature.
CHEMICAL HAZARD. Formamide. Exposure causes eye,
skin, and respiratory tract irritation. It is a possible developmental and birth defect
hazard. Read the MSDS, and follow the handling instructions. Wear appropriate
protective eyewear, clothing, and gloves.
ELECTRICAL HAZARD. Failure to ground the instrument
properly can lead to an electrical shock. Ground the instrument according to the
provided instructions.
For Additional
Information
Please see the Safety chapters in:
• The protocols for the template preparation, sequencing chemistry and/or
extension product purification you use.
• The user guides for the thermal cycler and DNA sequencer you use.

xiDNA Sequencing by Capillary Electrophoresis Chemistry Guide
Safety and EMC Compliance Information
Chemical Safety
Chemical Hazard
Warning
CHEMICAL HAZARD. Before handling any chemicals, refer
to the Material Safety Data Sheet (MSDS) provided by the manufacturer, and
observe all relevant precautions.
About MSDSs
Chemical manufacturers supply current Material Safety Data Sheets (MSDSs) with
shipments of hazardous chemicals to new customers. They also provide MSDSs with
the first shipment of a hazardous chemical to a customer after an MSDS has been
updated. MSDSs provide the safety information you need to store, handle, transport,
and dispose of the chemicals safely.
Each time you receive a new MSDS packaged with a hazardous chemical, be sure to
replace the appropriate MSDS in your files.
Chemical Waste Safety
Chemical Waste
Hazard
HAZARDOUS WASTE. Refer to Material Safety Data Sheets
and local regulations for handling and disposal.
Biological Hazard Safety
BIOHAZARD. Biological samples such as tissues, body fluids,
infectious agents, and blood of humans and other animals have the potential to
transmit infectious diseases. Follow all applicable local, state/provincial, and/or
national regulations. Wear appropriate protective equipment, which includes but is
not limited to: protective eyewear, face shield, clothing/lab coat, and gloves. All work
should be conducted in properly equipped facilities using the appropriate safety
equipment (for example, physical containment devices). Individuals should be
trained according to applicable regulatory and company/institution requirements
before working with potentially infectious materials. Read and follow the applicable
guidelines and/or regulatory requirements in the following:
• U.S. Department of Health and Human Services guidelines published in
Biosafety in Microbiological and Biomedical Laboratories (stock no. 017-040-
00547-4; http://bmbl.od.nih.gov)
• Occupational Safety and Health Standards, Bloodborne Pathogens (29
CFR§1910.1030; http://www.access.gpo.gov/ nara/cfr/waisidx_01/
29cfr1910a_01.html).
• Your company’s/institution’s Biosafety Program protocols for working
with/handling potentially infectious materials.
Additional information about biohazard guidelines is available at:
http://www.cdc.gov

xii DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Safety and EMC Compliance Information

1DNA Sequencing by Capillary Electrophoresis Chemistry Guide
1
Introduction to DNA Sequencing 1
This chapter covers:
DNA Sequencing Basics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2
Cycle Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
Capillary Electrophoresis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .9
Data Analysis. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .11
Automated DNA Sequencing Workflow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13

2 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
DNA Sequencing Basics
This section presents basic synthesis, replication, and sequencing principles that you
need to know in order to perform automated DNA sequencing by capillary
electrophoresis.
Cell Replication
The process of DNA synthesis and replication in a cell involves DNA helicase, DNA
polymerase, DNA template, and deoxynucleotides. DNA replication starts when
DNA helicase unravels the double-helix structure to expose single-stranded DNA
and form a replication fork. RNA primase introduces a primer that binds to the
single-stranded DNA. DNA polymerase then binds to the replication fork and starts
DNA synthesis by sequentially adding nucleotides to the 3′-hydroxyl end of the RNA
primer bound to the DNA template (Figure 1). The result is the creation of an
“extension product.”
Figure 1 DNA replication fork
The extension product grows in the 5′ to 3′ direction by forming a phosphodiester
bridge between the 3´-hydroxyl group at the growing end of the primer and the 5´-
phosphate group of the incoming deoxynucleotide (Kornberg and Baker, 2005)
(Figure 2).
RNA primer
Newly synthesized DNA
RNA primase
DNA helicase
DNA polymerase

3DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
9a
Figure 2 DNA strand synthesis and termination
The DNA sequence is copied with high fidelity because at each base on the DNA
template, DNA polymerase incorporates only the nucleotide that is complementary
to that base. Thymine (T) is complementary to adenine (A) and guanine (G) is
complementary to cytosine (C) because they can form hydrogen bonds with each
other (Figure 3).
Figure 3 Base complements
3′hydroxyl group on nucleotide allows
phosphodiester bridge formation
3′hydroxyl group on dideoxynucleotide
terminates DNA synthesis
Te m pl a t e
Extension product
O
P
O
NN
OO
–
O
HN
O
HO
O
P
O
O
–
O
NH
N
N
N
N
O
O
5´
H
H
5´
H
O
3´
N
N
N
N
HN
O
O
P
O
OO
–
O
O
O
P
O
3´
N
NH
H
3
C
O
O
O
–
O
H
5´
HO
5´
AT CG

4 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
History of Sanger
Dideoxy
Sequencing
The principles of DNA replication were used by Sanger et al. (1974) in the
development of the process now known as Sanger dideoxy sequencing. This process
takes advantage of the ability of DNA polymerase to incorporate 2′, 3′-
dideoxynucleotides, nucleotide base analogs that lack the 3′-hydroxyl group essential
in phosphodiester bond formation.
Sanger dideoxy sequencing requires a DNA template, a sequencing primer, DNA
polymerase, nucleotides (dNTPs), dideoxynucleotides (ddNTPs), and reaction buffer.
Four separate reactions are set up, each containing radioactively labeled nucleotides
and either ddA, ddC, ddG, or ddT. The annealing, labeling, and termination steps are
performed on separate heat blocks. DNA synthesis is performed at 37 °C, the
temperature at which the T7 DNA polymerase used has the optimal enzyme activity.
DNA polymerase adds either a deoxynucleotide or the corresponding 2′, 3′-
dideoxynucleotide at each step of chain extension. Whether a deoxynucleotide or a
dideoxynucleotide is added depends on the relative concentration of both molecules.
When a deoxynucleotide (A, C, G, or T) is added to the 3′ end, chain extension can
continue. However, when a dideoxynucleotide (ddA, ddC, ddG, or ddT) is added to
the 3´ end, chain extension terminates (Figure 2). Sanger dideoxy sequencing results
in the formation of extension products of various lengths terminated with
dideoxynucleotides at the 3′ end.
Electrophoresis
The extension products are then separated by electrophoresis. During
electrophoresis, an electrical field is applied so that the negatively charged DNA
fragments move toward the positive electrode. The speed at which a DNA fragment
moves through the medium is inversely proportional to its molecular weight. This
process of electrophoresis can separate the extension products by size at a resolution
of one base.

5DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Applied
Biosystems
Automated DNA
Sequencing
Applied Biosystems fluorescence-based cycle sequencing system is an extension and
refinement of Sanger dideoxy sequencing. Applied Biosystems automated DNA
sequencing generally follows this flow:
1. Template preparation (Chapter 3)
2. Cycle sequencing (Chapter 4)
3. Purification after cycle sequencing (Chapter 5)
4. Capillary electrophoresis (Chapter 6)
5. Data analysis (Chapter 7)
Cycle Sequencing
Process
Overview
Like Sanger sequencing, fluorescence-based cycle sequencing requires a DNA
template, a sequencing primer, a thermal stable DNA polymerase, nucleotides
(dNTPs), dideoxynucleotides (ddNTPs), and buffer. But unlike Sanger’s method,
which uses radioactive material, cycle sequencing uses fluorescent dyes to label the
extension products and the components are combined in a reaction that is subjected
to cycles of annealing, extension, and denaturation in a thermal cycler. Thermal
cycling the sequencing reactions creates and amplifies extension products that are
terminated by one of the four dideoxynucleotides (Figure 4). The ratio of
deoxynucleotides to dideoxynucleotides is optimized to produce a balanced
population of long and short extension products.
Figure 4 Example cycle sequencing reactions in a thermal cycler
PRODUCTS
Reaction
Mixture
Enzyme, dNTPs
ACGT
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
Denaturation
ExtensionAnnealing
Original
Template
A
C
C
G
T

6 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Advantages
There are many advantages to performing cycle sequencing, including:
• Protocols are robust, easy to perform, and effective for sequencing PCR
products.
• High temperatures reduce secondary structure, allowing for precise priming,
template annealing, and thorough extension.
• The same protocol can be used for double- and single-stranded DNA.
• Difficult templates, such as bacterial artificial chromosomes (BACs), can be
sequenced.
How Extension
Products Are
Labeled
Automated cycle sequencing procedures incorporate fluorescent dye labels using
either dye-labeled dideoxynucleotide triphosphates (dye terminators) or dye-labeled
primers (dye primers). Both chemistries use four different dyes. Because each dye
emits a unique wavelength when excited by light, the fluorescent dye on the
extension product identifies the 3′ terminal dideoxynucleotide as A, C, G, or T.
Dye Terminator
Chemistry
With dye terminator chemistry, each of the four dideoxynucleotide terminators is
tagged with a different fluorescent dye. One reaction is performed, containing the
enzyme, nucleotides, and all dye-labeled dideoxynucleotides. The products from this
reaction are injected into one capillary (Figure 5).
Figure 5 Diagram of dye terminator cycle sequencing
Advantages of dye terminator chemistry compared with dye primer chemistry:
• You can use unlabeled primers, which cost less than labeled primers.
• You can perform reactions in one tube.
• Reactions require fewer pipetting steps than dye primer reactions.
• False stops (fragments not terminated by a dideoxynucleotide) are not detected
because no dye is attached.
• BigDye terminators v1.1 and v3.1 and dRhodamine terminators are formulated
with dITP in place of dGTP to reduce peak compressions.
• dGTP BigDye terminators are formulated with dGTP for sequencing G-C-rich
templates or sequence motifs consisting of Gs and Cs.
Enzyme, dNTPs,
dye-labeled terminators
A
C
G
T
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
ANNEALING EXTENSION PRODUCTSDENATURATION

7DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Dye Primer
Chemistry
With dye primer chemistry, four separate tubes of sequencing primer are each tagged
with a different fluorescent dye. Four separate reactions are performed, each
containing the enzyme, nucleotides, a specific dye-labeled sequencing primer, and
either A, C, G, or T dideoxynucleotides. The products from these four reactions are
then combined and injected into one capillary (Figure 6).
Figure 6 Diagram of dye primer cycle sequencing
Advantages of dye primer chemistry compared with dye terminator chemistry:
• Dye primer chemistries generally produce more even peak heights than dye
terminator chemistries.
• Labeled primers are available for common priming sites. Custom primers can
also be labeled.
Cycle
Sequencing Kits
Applied Biosystems cycle sequencing kits available for both dye primer and dye
terminator chemistries:
•BigDye
®
Terminator v1.1 and v3.1 Cycle Sequencing Kits
• dGTP BigDye
®
Terminator v1.0 and v3.0 Cycle Sequencing Kits
• dRhodamine Terminator Cycle Sequencing Kits
•BigDye
®
Primer Cycle Sequencing Kits
See Appendix B, Parts List for product information.
Enzyme, dNTPs,
ddNTPs
A
A
A
TGCCA
C
C
A
C
CA
G
G
CCA
T
T
GC
T
C
G
A
CCAA
T
ANNEALING EXTENSION PRODUCTSDENATURATION

8 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Modified DNA
Polymerase
The cycle sequencing reaction is directed by highly modified, thermally stable DNA
polymerases. These enzymes have been carefully selected to allow incorporation of
dideoxynucleotides, to process through stretches of G-C-rich and other difficult
sequences, and to produce uniform peak heights. The modified DNA polymerases
are also formulated with a pyrophosphatase to prevent reversal of the polymerization
reaction (pyrophosphorolysis).
Emission Spectra
of Fluorescent
Dyes
The fluorescent dyes used in BigDye
®
terminators, BigDye
®
primers, and
dRhodamine terminators have narrower emission spectra and less spectral overlap
than the rhodamine dyes used in previous sequencing kits. As a result, the dyes
produce less noise. Figure 7 shows the normalized emission spectra and spectral
overlap of the four dyes in the BigDye Terminator Cycle Sequencing Kit.
Figure 7 Emission spectra of the four BigDye dyes, where Dye 1 = Big-d110,
Dye 2 = R6G, Dye 3 = Big-dTAMRA, and Dye 4 = Big-dROX
Dye 1 Dye 2 Dye 3 Dye 4
Normalized Emission Intensity
Wavelength (nm)

9DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Capillary Electrophoresis
Historically, DNA sequencing products were separated using polyacrylamide gels
that were manually poured between two glass plates. Capillary electrophoresis using
a denaturing flowable polymer has largely replaced the use of gel separation
techniques due to significant gains in workflow, throughput, and ease of use.
Fluorescently labeled DNA fragments are separated according to molecular weight.
Because you do not need to pour gels with capillary electrophoresis, you can
automate DNA sequence analysis more easily and process more samples at once.
Process
Overview
During capillary electrophoresis, the extension products of the cycle sequencing
reaction enter the capillary as a result of electrokinetic injection. A high voltage
charge applied to the buffered sequencing reaction forces the negatively charged
fragments into the capillaries. The extension products are separated by size based on
their total charge.
The electrophoretic mobility of the sample can be affected by the run conditions: the
buffer type, concentration, and pH; the run temperature; the amount of voltage
applied; and the type of polymer used.
Shortly before reaching the positive electrode, the fluorescently labeled DNA
fragments, separated by size, move across the path of a laser beam. The laser beam
causes the dyes on the fragments to fluoresce. An optical detection device on
Applied Biosystems genetic analyzers detects the fluorescence. The Data Collection
Software converts the fluorescence signal to digital data, then records the data in a
*.ab1 file. Because each dye emits light at a different wavelength when excited by the
laser, all four colors, and therefore all four bases, can be detected and distinguished
in one capillary injection (Figure 8).
Figure 8 Fluorescent sequencing compared with radioactive sequencing
One color
Four lanes
Four colors
One lane
T reaction
C reaction
G reaction
A reaction

10 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Available
Instruments
Applied Biosystems offers the following automated genetic analyzers (Table 1).
For more information about each instrument, refer to the appropriate instrument user
guide.
Table 1 Applied Biosystems genetic analyzers
Instrument
Number of
Capillaries
Capillary
Array
Length
‡§
(cm)
Polymer Type Sample Capacity
#
Applied Biosystems 3730xl DNA
Analyzer
96 36, 50 POP-7
™
96- and 384-well plates
Applied Biosystems 3730 DNA Analyzer 48 36, 50 POP-7
™
96- and 384-well plates
Applied Biosystems 3130xl Genetic
Analyzer
16 36, 50, 80 POP-7
™
POP-4
™
POP-6
™
96- and 384-well plates
Applied Biosystems 3130 Genetic
Analyzer
4 36, 50, 80 POP-7
™
POP-4
™
POP-6
™
96- and 384-well plates
Applied Biosystems 3100 Genetic
Analyzer
16 36, 50, 80 POP-4
™
POP-6
™
96- and 384-well plates
Applied Biosystems 3100-Avant Genetic
Analyzer
4 36, 50, 80 POP-4
™
POP-6
™
96- and 384-well plates
Applied Biosystems 310 Genetic
Analyzer
147, 61POP-4
™
POP-6
™
48 or 96 sample tubes
‡ 22-cm capillaries are not listed because they are not used for sequencing applications.
§ For multicapillary instruments (all but the 310 instrument), the capillary array length is the well-to-read length.
# Sample capacity is the number of samples or plate types the autosampler can accommodate.

11DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Data Analysis
Process
Overview
Data analysis software processes the raw data in the *.ab1 file using algorithms and
applies the following analysis settings to the results:
• Multicomponent analysis – Each fluorescent dye emits its maximum
fluorescence at a different wavelength, but there is some overlap in the emission
spectra (Figure 7 on page 8). Thus a signal generated primarily in one color
channel may yield a lower signal in an adjacent color channel. Multicomponent
analysis separates the four different fluorescent dye signals into distinct spectral
components by mathematically filtering fluorescence signal from dyes with
emission spectra overlap
• Basecalling – The selected basecaller processes the fluorescence signals, then
assigns a base to each peak (A, C, G, T, or N). If the KB
™
basecaller is used, it
also provides per-base quality value predictions, optional mixed base calling,
and automatic identification of failed samples (page 145).
• Mobility shift correction – The mobility file (page 145) corrects
electrophoretic mobility changes imposed by the presence of different
fluorescent dye molecules associated with differently labeled reaction extension
products (page 262). The mobility file also corrects for the differences between
the dye-to-nucleotide relationships in the raw data and the analyzed data.
• Quality value determination (QV) – If the KB basecaller is used for analysis,
the software assigns a QV for each base. The QV predicts the probability of a
basecall error. For example, a QV of 20 predicts an error rate of 1%. The quality
prediction algorithm is calibrated to return QVs that conform to the industry-
standard relationship established by the Phred software. If your pipeline
involves analysis with Phred software to assign QVs after the data is basecalled,
you can simplify your workflow and use the KB basecaller instead. The KB
basecaller can perform basecalling and assign QVs. Then, you can generate
phd.1 or *.scf files using the KB basecaller to integrate with your downstream
pipeline.
Analyzed sample data is displayed as an electropherogram, a sequence of peaks in
four colors. Each color represents the base called for that peak (Figure 9).
Figure 9 Example of electropherogram showing data analyzed with the KB
™
basecaller

12 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Software
Products
Table 2 lists Applied Biosystems software products for analyzing sequencing data.
For more detailed information, see “Analysis Software” on page 159.
Data Analysis
Options
Using Applied Biosystems Data Collection Software, you may analyze your
sequencing files automatically, immediately after the electrophoresis run, or
manually:
• Autoanalysis – With autoanalysis, the software applies analysis protocols to
sequencing files immediately after Data Collection Software collects the data
from the instrument. The analyzed data is saved in the sequencing file. You can
review the analyzed data using Sequencing Analysis Software, SeqScape
Software or MicroSeq ID Analysis Software.
• Manual analysis – With manual analysis, you obtain the sequencing files from
the computer connected to the instrument, then move or copy the files to another
computer that has any of the Applied Biosystems analysis software installed. To
perform analysis, you manually apply the analysis protocols to the sequencing
files, start analysis, and save the analyzed data.
Table 2 Applied Biosystems analysis software
Product Suggested Use
Sequence Scanner
Software
Viewing or editing traces, evaluating trace quality, making trace
QC reports
Sequencing Analysis
Software
Viewing or editing traces, evaluating trace quality, making trace
QC reports, Re-analyze traces
Variant Reporter™
Software
Mutation detection, SNP discovery and validation
SeqScape
®
Software Mutation detection, SNP discovery and validation, sequence
comparison, typing
MicroSeq
®
ID Analysis
Software
With MicroSeq ID Kit, for bacterial and fungal identification

13DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Automated DNA Sequencing Workflow
DNA Template
Preparation
(Chapter 3)
1. Prepare DNA template from plasmid, DNA fragment, BAC or YAC. Or prepare DNA template
by PCR.
2. Design primers
3. Clean up templates.
4. Examine DNA quality.
5. Determine DNA quantity.
Examples of DNA Template Preparation Output:
•PCR products
• Plasmid DNA
•Genomic DNA
• Bacterial artificial chromosomes (BACs)
• Yeast artificial chromosomes (YACs)
•Cosmids
Cycle
Sequencing
(Chapter 4)
1. Select the sequencing chemistry.
2. Prepare cycle sequencing reactions.
3. Run sequencing reactions in a thermal
cycler.
Applied Biosystems Cycle Sequencing Kits:
• BigDye Terminator v1.1 and v3.1 kits
• dGTP BigDye Terminator v1.0 and v3.0 kits
• BigDye Primer kits
• dRhodamine Terminator kits
Cycle Sequencing Output:
Extension
Product
Purification
(Chapter 5)
Purify extension products using one method:
•BigDye
®
XTerminator
™
Purification
• Ethanol precipitation
• Spin-column purification
• Alternative cleanup procedures
After purification, prepare samples for electrophoresis
Extension Product Purification Output:
Purified dye terminator products or purified dye primer products
F1 F2 F3 F4
1 2 3
4 5 6
7 8 9
ENTER
STOP
0 CE
F5
GeneAmp
®
PCR System 9700
POWER
G
e
n
e
A
m
p
®
P
C
R
S
y
s
t
e
m
9
7
0
0
9700 thermal
cycler
9800 thermal
cycler
Veriti
®
thermal
cycler
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
or
Dye terminator products
Dye primer products
14 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 1 Introduction to DNA Sequencing
Capillary
Electrophoresis
(Chapter 6)
1. Prepare the instrument.
2. Set up the plate record in Data Collection
Software:
– Results group
– Instrument protocol
– Analysis protocol
3. Load samples.
4. Perform the run.
Capillary Electrophoresis Output:
Applied Biosystems instruments:
Data Analysis
(Chapter 7)
1. Apply analysis protocols:
– Basecaller
– Mobility file
2. Run analysis.
3. Review the data.
Applied Biosystems software:
• Sequencing Analysis Software
• Variant Reporter
TM
Software
• SeqScape Software
• Sequence Scanner Software
•MicroSeq
®
ID Analysis Software
Data Analysis Output:
*.ab1 file
3730/3730xl analyzer
3100/3100-Avant analyzer
ABI PRISM
1
2
3
4
5
B-D
310 analyzer
3130/3130xl analyzer
Analyzed project in Variant Reporter Software
Analyzed project in
SeqScape Software
Analyzed sample file in
Sequencing Analysis
Software

15DNA Sequencing by Capillary Electrophoresis Chemistry Guide
2
Applications Overview 2
This chapter covers:
DNA Sequencing Applications and Approaches . . . . . . . . . . . . . . . . . . . . . . . . . . .16
De Novo Sequencing of Genomes. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16
Resequencing. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20
Epigenetics . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23
Microbial Analysis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .27

16 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
DNA Sequencing Applications and Approaches
DNA sequencing can be used for a variety of applications, including:
• De novo sequencing of genomes
• Detection of variants (SNPs) and mutations
• Biological identification
• Confirmation of clone constructs
• Detection of methylation events
• Gene expression studies
• Detection of copy number variation
Factors in
Selecting an
Approach
The approach used to sequence a target sample depends on several factors:
• Length of the target – Target length ranges from determining the sequence of a
single base to a large genome.
• Complexity of the sample – Samples can range from a single homogenous
sample source to a highly complex mixed sample that includes only a small
amount of the target sequence mixed in a high background.
• Number of samples – The number of samples ranges from a single sample or
thousands of samples.
• Prior knowledge of the targeted sequencing region – Knowledge of the
region ranges from none (de novo sequencing technologies) to having a full
reference (resequencing).
De Novo Sequencing of Genomes
De novo sequencing is used for the generation of the DNA sequence of a DNA
molecule without any prior information about the sequence. For genome projects, an
extremely high throughput level and high-end robotics are required in order to
accommodate the sequencing workflow.
De novo whole or partial genome sequencing may be addressed through a variety of
general approaches. Each of these broad strategies has been developed to include a
number of specific techniques. The selection of an individual method relies on a
number of different factors that include: genome size; whether the project involves
an entire genome or targets some genome fraction (i.e. a specific chromosome); the
desired coverage; and the resources available. The general approach selected will
define most of a project's elements beginning with vector selection and ending with
sequence data analysis and assembly.
This section includes a brief overview of the general approaches. Guidance for
conducting individual protocols may be obtained from commonly available sources.
For de novo sequencing using capillary electrophoresis, the target DNA is
fragmented and cloned into a viral or plasmid vector. Cloning provides amplification
of the target DNA (by bacterial growth) and allows sequencing primers to bind to
known sequence in the vector and extend the sequence into the unknown target DNA.

17DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Genomic DNA fragments longer than 50 kb can be less efficient targets for PCR
amplification than shorter genomic DNA fragments (QIAGEN Genomic DNA
Handbook, 1999-2000). If you isolate long DNA fragments, you may need to shear
the DNA by vortexing it for 3 to 5 minutes or by passing the preparation several
times through an 18-gauge needle attached to a sterile syringe. See page 34 for more
information.
Template Amplification Using Large Insert Vectors
Large-target DNA is cloned into Bacterial Artificial Chromosomes (BAC) with
insert sizes ranging from 100 to 300kb. The BACs are then subcloned into smaller
vectors that are more suitable for Sanger sequencing, with typical insert sizes of 1 to
10kb. Bacterial clones are isolated and grown in media, the plasmid or phage DNA is
extracted, and the purified DNA template is used for forward and reverse sequencing
reactions.
Figure 10 Proposed de novo sequencing workflow
Clones with small inserts can be completely sequenced using sequencing primers
that hybridize to either end of the insert, then sequencing in the forward and reverse
directions.
De Novo Sequencing Methods
Various workflow strategies are used to perform de novo sequencing. They include
but are not limited to:
• Shotgun sequencing
• Primer walking
• Using transposons to randomly prime sites for sequencing
• Nested deletions
• PCR amplification of template
• mRNA sequencing
A
C
C
G
T
A
C
C
G
A
C
C
A
CA
Isolate bacterial colonies
Prepare plasmid DNA
Run sequencing reactions
Perform cleanup
Perform capillary electrophoresis
Analyze data

18 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
• Expressed Sequence Tags (EST)
Shotgun
Sequencing
For large target DNA, a time-efficient method of sequencing is to randomly shear the
DNA into smaller pieces (0.5 to 1.5 kb) by enzymatic digestion or physical shearing.
These shotgun fragments are subcloned into vectors, transformed into bacteria and
isolated as colonies. The colonies are inoculated into media and grown overnight.
The vector DNA is extracted and then sequenced from standard priming sites in the
vector (Figure 11).
The shotgun method replaced directed sequencing, where a physical map of the
clones and subclones was created before sequencing to serve as a guide to assemble
the sequence traces. Shotgun sequencing does not require prior information about the
sequence, and it can be used for DNA molecules as large as entire chromosomes.
Figure 11 Shotgun sequencing
To ensure full coverage of double-stranded template DNA, it may be necessary to
sequence a region 7 to10 times. A BAC with a 100 kb insert would require about
1500 subclones (500 to 1000 bp) for complete coverage. A cosmid (40 kb insert)
may require about 250 to 500 subclones.
Primer Walking
An alternative to shotgun sequencing is primer walking. Following the initial
sequencing determination, primed from a region of known sequence, subsequent
primers are designed to hybridize to 3' regions, determined in previous steps
(Figure 12). These primers then serve as sequencing start point to establish an
additional >500 bp of sequence data. New primers are synthesized for the newly
established sequence in the template DNA, and the process continues.
A
C
C
A
CA
A
C
C
A
CA
A
C
C
A
CA
A
C
C
A
CA
A
C
C
A
CA
A
C
C
A
CA
Extract DNA
Perform DNA fragmentation
Clone into vectors
Transform into bacteria; grow, isolate vector DNA
Sequence the library
Assemble contiguous fragments

19DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Figure 12 Primer walking
The primary advantage of primer walking is that extensive subcloning is not
required. The amount of overlap or coverage required is also decreased because the
direction and location of the new sequence is known, substantially decreasing the
effort needed to assemble the final sequence. The primary disadvantage of primer
walking is the amount of time required for each step in the primer walk and the need
to design a robust primer for every step. Primer walking is often used to fill gaps in a
sequence that has been determined by shotgun cloning.
Random
Sequencing
Priming Sites by
Transposo ns
An alternative to subcloning is the random introduction of a jumping DNA element
(transposon) into the target DNA. The target DNA is grown in a bacterial host with
the appropriate element and a transposase gene. The vector DNA isolated from the
transposase-positive strain and is used to transform the transposase-negative strain.
The transposable element provides a known hybridization site for a sequencing
primer, allowing sequencing and assembly of the target DNA from multiple internal
locations (Figure 13).
Figure 13 Transposon sequencing
Nested Deletions
Nested deletion strategies, with exonucleases or with restriction enzymes, help bring
unknown DNA regions closer to the sequencing priming sites (Figure 14).
Figure 14 Nested-deletion sequencing
Primer 1
New sequence
Primer 3
Primer 2
Primer
New sequence
Transposable element
Primer 3
Primer
New sequence
Deletion
Larger deletion

20 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
PCR
Amplification of
Template
All the above methods depend on template amplification by growth of bacteria. The
advantage of cloning and bacterial growth is that it makes isolation and amplification
of pure templates relatively easy. The primary disadvantage of bacterial growth is the
time and effort required to produce a template. Typically, two days are required for
transformation and isolation of colonies, bacterial growth and plasmid DNA
preparation. It may be possible to shorten the overall time using single-molecule
PCR. The fragmented genomic DNA is ligated to end-linkers and then diluted to a
concentration of approximately one molecule per tube. The single molecule is then
amplified by PCR and sequenced. Sequences that indicate mixed templates are
discarded and “pure” sequences are assembled by standard procedures.
mRNA
Sequencing
In addition to genomic DNA sequencing, sequencing of mRNA has been used to
understand gene structure and to develop prediction rules for annotation of introns
and exons annotations in genomic DNA sequence. Mature mRNA sequences are
isolated from an organism, converted to cDNA sequences by reverse transcriptase,
and cloned as libraries. The inserts in these libraries are then completely sequenced.
Significant effort is expended to create clones that encompass the complete mRNA
transcript and that also capture alternative forms of a transcript. To ensure that a
complete mRNA is produced in the clone, cDNA is synthesized from the RNA using
random primers in addition to the poly A primers. cDNA sequences are also
extended to the 5′-end of a transcript by methods like rapid amplification of cDNA
ends (5′-RACE) with mRNA specific primers. The overlapping sequences are then
assembled to generate a super transcript that potentially includes all known
transcripts of a specific gene.
Expressed
Sequence Tags
(EST)
The mRNA component of a biological sample may also be sequenced to aid in the
identification of active genes in a tissue. The mRNA from a tissue is cloned into a
vector. The vector is used to transform bacteria, followed by isolation of bacterial
colonies, bacterial growth, DNA extraction, and sequencing. Typically, each clone is
sequenced just once to produce a “single-pass” sequence tag of about 300 to 800
bases. These tags provide information about the gene content of a tissue under the
conditions in which the tissue was harvested. The GenBank database, dbEST,
contains sequence data and related information on sequences from a great many
organisms.
Resequencing
Resequencing is defined as sequencing of DNA molecules followed by comparison
to a known or reference sequence. Resequencing or directed sequencing is used for
the discovery of sequence variants usually associated with a phenotypic change, for
determining evolutionary changes, and/or for biological identification. Resequencing
may be focused on coding regions of genes implicated in disease, or it may target the
whole genome for the discovery of SNPs and other sequence variations between
individuals. Comparative sequencing is usually defined as sequencing a specific
region in different species or subspecies to identify highly conserved regions. Highly
conserved regions are usually indicative of conserved function between the species,
and they can be used to associate gene areas with conserved phenotype.

21DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Resequencing is often carried out by amplifying a specific region of the genome by
PCR and then sequencing the PCR fragment from both directions to generate a high-
quality DNA sequence. Multiple DNA samples are processed simultaneously in
micro-well plates, and the sequence traces are compared directly with each other to
establish sequence variants.
A resequencing project may involve PCR amplifying and sequencing ten to hundreds
of genes, with about 20 amplicons per gene for each individual genomic DNA
sample.
High sensitivity, that is, a very high percentage of true positives and very low
percentage of false negatives, is required to deliver complete mutation detection by
revealing sequence variants in the sample, compared with a reference sequence. High
sensitivity is also required to detect a small percentage of change in an
overwhelmingly normal background (as in mixed samples such as cancer isolates or
pooled DNA samples). For this reason, PCR fragments are sequenced
bi-directionally to achieve greater than 99% accuracy.
Note: A mutation is a change in the sequence of the test sample when compared with
the sequence of a reference. A polymorphism is a mutation that occurs in a
substantial proportion of the population (typically greater than 1%).
Various strategies for resequencing include:
• Using the PCR primer as the sequencing primer
• Designing PCR primers with a sequencing tail
• Using nested (internal) sequencing primers
• Bisulfite sequencing for methlyation analysis
PCR Primer as
the Sequencing
Primer
Using the PCR primer as the sequencing primer (Figure 15) decreases the need for
synthesizing specific sequencing primers, because an aliquot of the primers for PCR
can be used for setting up the sequencing reaction. The disadvantage is that separate
sequencing master mixes must be prepared for each sequencing direction and for
each amplicon, increasing the number of pipetting steps and the possibility of error.
Figure 15 Sequencing with PCR primers
Forward sequencing primer
Reverse sequencing primer
PCR
Sequencing
PCR primer
DNA template
PCR template

22 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
PCR Primers with
a Sequencing Tail
For most large projects, it has become customary to include a standard primer tail on
the PCR primers to simplify sequencing set-up (Figure 16). The most common tail is
the sequence known as the M13 sequence because it was initially used for
sequencing clones constructed in the single-stranded bacteriophage M13. Most
sequence service providers provide the standard M13 primer at no additional cost.
Figure 16 Sequencing with PCR primer and standard primer tail
Potential disadvantages of using tailed PCR primers are the greater challenge in
designing primers with a tail and the need for higher quality oligonucleotides due to
the increase in primer length.
Nested (Internal)
Sequencing
Primers
Designing PCR primers for amplification of closely related genes/pseudogenes can
be challenging, because the PCR reaction may produce a mixture of PCR fragments.
These fragments can be resolved by using an internal sequencing primer that is
specific to only one of the PCR fragments.
Figure 17 Sequencing with subtype-specific tail and PCR primer tail
PCR primer
with tail
Forward sequencing primer
Reverse sequencing primer
PCR
Sequencing
Tail sequence for
sequencing primer
DNA template
PCR template
Nested sequencing primer
PCR
Sequencing
PCR primer
PCR primer
DNA template
PCR template

23DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Nested sequencing primers are used in the primer walking method discussed in the
de novo sequencing section (page 18), and also for genotyping applications such as
those served by the SNaPshot technology. The nested primer is designed to bind at
the n-1 position of a suspected mutation. The primer is extended in the presence of
the four fluorescent-labeled ddNTPs. Incorporation of a specific NTP (specific
color) indicates the presence of that base on the template.
For more information, refer to the ABI PRISM
®
SNaPshot
®
Multiplex Kit Protocol
(PN 4323357A).
Epigenetics
Methylation of DNA in vertebrate cells results in the regulation of gene expression
and is responsible for normal (and abnormal) cellular differentiation pathways. This
second code, the DNA methylation pattern, is an additional layer of information
superimposed on the DNA code that determines many phenotypic attributes. Though
the DNA code is largely unchanging, DNA methylation patterns do change in
response to spatial, temporal, and environmental cues. To accurately describe the
phenotype, the methylation pattern of DNA must be determined.
Selective gene inactivation has been shown to result from the DNA methylation of
cytosine in the promoter regions.
A methylation-specific cystosine is often associated with a guanine residue as a CpG
dinucleotide. Multiple CpG islands (that is, regions of < 500 bp with GC content of >
55%) have been identified around regulatory regions of genes.
Methylation of a CpG residue can be determined by treating genomic DNA with
sodium bisulfite that converts non-methylated cytosine to uracil, while methylated
cytosine is protected from bisulfite conversion (Figure 18). Comparing the sequence
of bisulfite-converted DNA with untreated DNA clearly indicates the presence of
methylated C residues, because they appear as C in bisulfite-converted DNA. Non-
methylated C is converted to U (and to T in the sequencing reaction), so it appears as
T.

24 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Figure 18 Conversion of methylcytosine to cytosine and non-methlylated
cytosine to uracil
In principle, there are two approaches to methylation, depending upon the available
information and the research goals: methylation-specific PCR or bisulfite-specific
PCR. A researcher performs bisulfite treatment in order to transform an epigenetic
event to a detectable, permanent genetic change in vitro, because the original
methylation is lost during PCR.
Comparison of sodium-bisulfite treated DNA sequences with sequences obtained
from untreated genomic DNA allows the precise identification of all methylated
cytosines within a long stretch of DNA (Figures 19 and 20) (Boyd, 2004).
Figure 19 DNA sequence from untreated DNA. Arrows show location of
unmethlyated cytosine located before guanine. After bisulfite treatment,
unmethlyated cytosine is converted to T.
GCGGTAGCGATGAGGGTTTGGTTAGCGTCGCGGCGCGG
160150 170 180

25DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Figure 20 DNA sequence from bisulfite-treated DNA. Arrows show location of
non-methylated cytosine converted to thymine after bisulfate sequencing.
Comparison of peaks from bisulfite sequencing and non-bisulfite sequencing is not
quantitative. For more quantitative analysis of methlyation, perform fragment
analysis.
Two options are available for collecting methylation sequencing data. Both options
require bisulfite conversion and PCR amplification, but in one method, the PCR
fragments are sequenced directly, while in the other method the fragments are cloned
and then the clones are sequenced. The cloning method has been applied to genome
wide sequence analysis of methylation (Meissner, 2005).
In the workflow shown in Figure 21, bisulfite sequencing without a cloning step (on
the right) is compared with regular sequencing (on the left).
UTUUTAUTUATUAUUUTTTUUTTAUTUTTUTUUTUTUUU
160 170 180 19

26 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Figure 21 Steps for bisulfite sequencing compared with regular sequencing
workflow
For More Information
The following Applied Biosystems documents provide more information about
bisulfite sequencing:
• Applied Biosystems methylSEQr
™
Bisulfite Conversion Kit Protocol
(PN 4374710)
• Advances in Capillary Electrophoresis-Based Methods for DNA Methylation
Analysis Workflow Guide (PN 106BR14-01)
• Methylation Analysis Using FFPE Samples (PN 137AP07-01) (Available from
the Genetic Analysis section of the Applied Biosystems web site)
Analysis
DNA extraction
Sequencing Template preparation Sample preparation
Denature
Bisulfite
Cleanup
PCR
Cleanup
CE
Cleanup
Quantitate
(optional)
Cycle
sequencing
PCR
Cleanup
CE
Cleanup
Quantitate
(optional)
Primer
design
Primer
design
Cycle
sequencing
Analysis
Fragment Analysis
PCR
CE

27DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Microbial Analysis
Microbial analysis is used to identify and classify bacterial and fungal organisms at
the species level. Molecular epidemiology, population structure studies and studies of
pathogenic bacterial species use this information.
Strategies used to perform microbial analysis include:
• Multi Locus Sequence Typing (MLST) comparison of housekeeping genes to
library standards
• MicroSeq
®
ID Analysis Software comparison of ribosomal sequences to library
standards
Multi Locus
Sequence Typing
(MLST)
Multi Locus Sequence Typing (MLST) is a nucleotide sequence-based approach for
the unambiguous characterization and subspeciation of bacteria isolates and other
organisms. The technique identifies alleles by direct DNA sequencing of fragments
of housekeeping genes from known microorganisms. It is much more precise than
indirect methods, which identify microorganisms on the basis of the electrophoretic
mobility rates of large DNA fragments of gene products.
Figure 22 Multi Locus Sequence Typing (MLST) workflow
Isolate bacterial DNA samples
Amplify housekeeping genes
with MLST primers using PCR
Cycle sequence amplified genes
using BigDye Terminator v 1.1
Obtain reference sequence from MLST web site
and import into SeqScape® software
Collect sequencing data and
analyze with SeqScape software
Obtain allelic profile from library match
Identify sequence type using
allelic profile at MLST web site

28 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
The MLST technique characterizes isolates of bacterial species, using internal
fragment sequences of approximately 450 to 500 bp from several housekeeping
genes. Both strands of the fragments can be accurately sequenced by capillary
electrophoresis. The various sequences present in a bacterial species for each
housekeeping gene are specified as distinct alleles. For each isolate, the alleles at all
loci define the allelic profile or sequence type (ST). This sequence type can be used
to query the database in the MLST web site at
saureus.mlst.net/sql/allelicprofile_choice.asp.
MicroSeq
®
ID
Analysis Software
Microbial identification based on ribosomal RNA gene sequencing is used to
identify microbial species including bacteria, yeasts, molds, and fungi. Bacteria are
identified by sequencing the universal 16S ribosomal RNA (rRNA) gene, which
forms the basis for bacterial taxonomic classification in Bergey's Manual. Fungi are
identified by sequencing the D2 region of the 26S rRNA gene. These sequences are
compared to validated sequences in the microbial libraries. MicroSeq ID Analysis
Software automatically matches unknown samples to the selected percent match or
the closest match in the library. The list of closest matches is ranked according to
genetic distance from the sample, displayed with a phylogenetic tree.
Figure 23 MicroSeq
®
ID Analysis Software, showing the top match of an
unknown sequence to the library
Libraries provided with the MicroSeq ID Analysis Software include entries for over
1,700 bacterial species, including gram-negative non-fermenters, Bacillus,
Coryneforms, Mycobacteria, and Staphylococcus. The library for fungal species
includes over 1,000 entries. Users can create libraries for species of interest and add
sequences from new or proprietary strains.

29DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview
Figure 24 MicroSeq
®
ID Analysis Software, showing the alignment of the
forward and reverse sequences of a sample (with electropherograms) to the
consensus sequence generated by the software (at the top). The consensus
sequence is compared to the validated library.
Figure 25
For more information, see the Applied Biosystems MicroSeq
®
ID
Analysis Software Version 2.0 Getting Started Guide (PN 4364623).

30 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 2 Applications Overview

31DNA Sequencing by Capillary Electrophoresis Chemistry Guide
3
DNA Template Preparation 3
This chapter covers:
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Preparing Vector-Based DNA Templates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Preparing Genomic DNA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34
Preparing PCR DNA Templates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Primer Design and Quantitation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .38
Purifying PCR Products for Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .41
DNA Template Quality . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44
DNA Template Quantity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .45
Preparing Templates for Bisulfite Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . .46
32 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Overview
The DNA purification method used can affect the quality of the template. This
chapter provides:
• Recommendations, considerations, and lists of commercial products for
preparing the following types of DNA templates:
– Vector-based templates (page 33)
– Genomic DNA (page 34)
– PCR DNA templates (page 37)
• Procedure for cleaning up templates (page 44)
• Guidelines for evaluating DNA quality (page 44)
• Guidelines for quantitating the DNA (page 45)
• Procedures for performing bisulfite sequencing (page 46)
Workflow
DNA Template Preparation
1. Prepare DNA template from plasmid, DNA fragment, BAC or YAC. Or prepare DNA
template by PCR.
2. Design primers
3. Clean up templates.
4. Examine DNA quality.
5. Determine DNA quantity.
Examples of DNA Template Preparation Output:
•PCR products
• Plasmid DNA
•Genomic DNA
• Bacterial artificial chromosomes (BACs)
• Yeast artificial chromosomes (YACs)
•Cosmids
Cycle Sequencing (Chapter 4)
Extension Product Purification (Chapter 5)
Capillary Electrophoresis (Chapter 6)
Data Analysis (Chapter 7)

33DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Preparing Vector-Based DNA Templates
Host Strain
Variability
The host strain that you use to clone the template can impact template quality. If you
plan to use a commercial template preparation kit, contact the vendor for information
about host strains that work well with that kit.
For more information about host strain effects see the QIAGEN Guide to Template
Purification and DNA Sequencing (2nd edition). To obtain a copy of this guide, visit
the QIAGEN web site (www.qiagen.com) or contact your local QIAGEN office.
Preparing Single-
Stranded DNA
One method involves preparing single-stranded DNA templates from M13 phage by
centrifugation and PEG precipitation of the phage particles. For more details, see
Sambrook and Russell (2001).
For more information about preparing single-stranded DNA, see the QIAprep M13
Handbook.
Preparing
Plasmid DNA
When purifying recombinant plasmids from bacteria, plate out the transformants to
obtain isolated colonies. Select a single colony and streak it out on a plate. Select an
isolated colony from the second plate to obtain plasmids with the desired insert.
The optimal method for preparing a particular plasmid depends on the particular
bacterial strain and the yield of each construct. General methods:
• Alkaline lysis
• Cesium chloride (CsCl) purification (for low copy and high molecular weight
plasmids)
• Simple boiling prep (not recommended for low copy number plasmids)
For more information about preparing plasmid DNA, see Sambrook and Russell
(2001).
Preparing BAC
DNA Templates
With larger DNA targets such as bacterial artificial chromosomes (BACs), the
quality of DNA template is especially important to the success of the sequencing
reaction. General methods that have given good sequencing results:
• Alkaline lysis, with extra phenol extraction and isopropanol precipitation if very
clean DNA is desired (Marra et al., 1996)
• Cesium chloride (CsCl) banding
For other BAC DNA preparation protocols, refer to the following:
• ABRF DNA Sequencing Research Group Electronic Posters. 1996-2008.
www.abrf.org/index.cfm/group.show/DNASequencing.28.htm#R_2.
Accessed 30 April 2008.
• Centre National de Séquençage (CNS, or Génoscope).
www.cns.fr/externe/arabidopsis/protoBAC.html.
Accessed 30 April 2008.
• University of Oklahoma Advanced Center for Genome Technology:
www.genome.ou.edu/DblAcetateProcV3.html.
Accessed 1 May 2008.
• Applied Biosystems Application Note: A Workflow for Obtaining High Quality
Sequencing Data from Bacterial Artificial Chromosome (BAC) DNA
(PN 107AP05-01)

34 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Commercial
Products
Table 3 lists commercial products that you can use to prepare vector-based DNA
templates. This is not an exhaustive list. Applied Biosystems makes no specific
recommendations on the use of these products. Follow the manufacturer’s
instructions to prepare the DNA templates for cycle sequencing.
Preparing Genomic DNA
Genomic DNA (gDNA) can be prepared from humans, animals, cell cultures, plants,
or fosmids. There are several options for preparing genomic DNA. To obtain enough
copies for fluorescent DNA sequencing, isolate the genomic DNA, then perform
PCR amplification of the specific target sequence before proceeding with cycle
sequencing.
Note: Applied Biosystems does not recommend direct sequencing of gDNA, with
the exception of bacterial gDNA.
Table 3 Commercial products for preparing vector-based DNA
Product Source Purpose
CosMCPrep
®
Kit Agencourt Bioscience
Corporation
To purify high-copy and low-copy plasmids, BACs,
cosmids, and fosmids
NucleoBond Plasmid
Purification Kits
Clontech Laboratories To purify high-copy and low-copy plasmids, cosmids,
BACs, PACs, and YACs
Aurum
™
Plasmid
Purification Kits and
Quantum Prep Plasmid
Purification Kit
Bio-Rad Laboratories To purify plasmid DNA
ProPrep
™
BAC 96 Biotech Support Group To miniprep BAC DNA in 96-well format
SeqPrep
™
Plasmid Prep Kit Edge Biosystems To purify plasmid DNA in 96-well or 384-well formats
illustra TempliPhiDNA
Sequencing Template
Preparation Kit
GE Healthcare To amplify single- or double-stranded circular DNA
templates from bacterial cells containing a plasmid, purified
plasmids, or intact M13 phage
ChargeSwitch
®
-Pro Plasmid
Miniprep Kit
Invitrogen To purify small-scale plasmid DNA
Montage Plasmid
Miniprep
HTS
96 Kit
Millipore To purify plasmid or BAC DNA in 96-well format
PSIΨClone BAC DNA Kits Princeton Separations To purify BAC DNA
Wizard
®
Plus Minipreps
DNA Purification Systems
Promega To purify plasmid DNA
Wizard SV 96 and SV 9600
Plasmid DNA Purification
Systems
Promega To purify plasmid DNA
QIAprep
®
Spin Miniprep Kits QIAGEN To purify plasmid DNA in 8-well or 96-well formats
GenElute
™
Plasmid
Miniprep Kit
Sigma-Aldrich To purify small-scale plasmid DNA

35DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Source Material
Considerations
Many sources of tissue can be used as starting points for the isolation of genomic
DNA or RNA, including fresh venous blood, anticoagulated blood (either EDTA,
citrate, or heparin), frozen blood, bone marrow, cultured cells, buccal scrapes, solid
organ biopsies, and paraffin-embedded tissue.
Consider the following when planning to perform cycle sequencing using genomic
DNA:
• Tissue type and amount – The type of source tissue and the amount available
can influence the effectiveness or sensitivity of PCR amplification.
• Heparin – Heparin may weaken or completely inhibit amplification during
PCR (Holodniy et al., 1991). Only a few methods that reverse the effect of
heparin have proven to be successful (Beutler et al., 1990). The manufacturers
of the Dynabeads DNA Direct Kit (Dynal, A.S.) and the QIAamp Blood Kit
(QIAGEN, GmbH) report that the genomic DNA obtained from heparin blood
using their products is ready for PCR amplification, because of the successful
removal of heparin after the washing steps in these protocols.
• Paraffin-embedded tissue – Paraffin-embedded tissue provides numerous
challenges to successful PCR and sequencing of the PCR products. Consider the
following:
– The fixative used
– The length of time of fixing
– The age of the block
– The yield of DNA obtained
– The amount of degradation of the DNA in the paraffin
– The presence of any PCR inhibitors in the isolated DNA (Jackson et al.,
1991)
These issues may significantly influence the size of the product that can be
amplified and the reproducibility of amplification from one sample to another.
Even if the fragment successfully amplifies, products contributed from the
template preparation can result in decreased signal or increased background
fluorescent noise from the sequencing reactions. Applied Biosystems
recommends the RecoverAll
™
Total Nucleic Acid Isolation Kit for FFPE
Tissues from Ambion (PN AM1975).
• RNA as the starting material – If you use RNA as your starting material via
RT-PCR (for example, RNA viruses, RNA transcripts), apply special
considerations. One allele might express differently from another or the gene
may not be expressed at all in some tissues. Also, elimination of RNases is
absolutely critical. For more information, see Sambrook and Russell (2001) or
go to www.ambion.com/techlib and search for “RNA isolation”.
Length of
Genomic DNA
Fragments
Genomic DNA fragments longer than 50 kbp can be less efficient targets for PCR
amplification than shorter genomic DNA fragments (QIAGEN Genomic DNA
Handbook). If you isolate long DNA fragments, you may need to shear the DNA by
vortexing it for 3 to 5 minutes or by passing the preparation several times through an
18-gauge needle attached to a sterile syringe.

36 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Methods
Noncommercial methods typically involve lysing the cells with lysozyme, alkali, or
detergents, then removing proteins and other contaminants by Proteinase K digestion
or phenol/chloroform extraction. Using Proteinase K is advantageous because the
enzyme destroys nucleases that can significantly reduce the molecular weight of the
DNA. However, the Proteinase K activity must be eliminated before PCR (Sambrook
and Russell, 2001).
Commercial
Products
Table 4 contains commercial products that you can use to prepare genomic DNA.
This is not an exhaustive list, and you may have found other products to work.
Applied Biosystems makes no specific recommendations on the use of these
products. Follow the manufacturer’s instructions to prepare the DNA templates for
cycle sequencing.
Commercial Products for Preparing RNA or cDNA
Information on a multitude of DNA and RNA purifications products offered by
Applied Biosystems is available at www.appliedbiosystems.com.
Browse from the home page by clicking Products DNA/RNA Purification.
For more information and to order, select the product line of interest.
Table 4 Commercial products for preparing genomic DNA
Product Source Purpose
MELT™ Total Nucleic Acid
Isolation System
Ambion
(PN AM1983)
To purify genomic DNA from tissue samples
RecoverALL™ Total Nucleic
Acid Isolation for FFPE
Tissues
Ambion
(PN AM1975)
To purify genomic DNA from FFPE tissue
TRI Reagent
®
Ambion
(PN AM9738)
To isolate total RNA or RNA, DNA, and protein from a variety
of fresh or frozen biological tissue or cells
NucPrep
®
DNA Isolation
Chemistry Starter Kit
Applied Biosystems
(PN 4340274)
To purify genomic DNA from cultured and fixed calls, gram-
negative bacteria, plant and animal tissue, and whole blood
and bone marrow cells
BloodPrep
®
DNA Starter Kit Applied Biosystems
(PN 4346860)
To purify genomic DNA directly from whole fresh and frozen
blood or isolated blood cells of many animal species, and
from tissue-cultured cells or buccal swab samples
Bacterial Genomic DNA
Purification Kit
Edge Biosystems To purify genomic DNA from bacterial cultures
PUREGENE
®
DNA Purification
Kits
QIAGEN To purify high molecular weight genomic DNA
QIAamp
®
DNA Blood
Mini/Midi/Maxi Kits
QIAGEN To purify genomic DNA from blood and blood-derived
samples

37DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Preparing PCR DNA Templates
This section provides information about preparing PCR products for sequencing, but
it is not intended to be a detailed guide to PCR amplification. For general
information on PCR amplification, refer to the product inserts included with
Applied Biosystems PCR reagents.
PCR Strategies
Because cycle sequencing involves many cycles of template denaturation and
extension, adequate signal is produced in the sequencing reaction. In selecting the
strategy for generating PCR DNA templates to be used for cycle sequencing,
consider specificity and yield. Use Applied Biosystems PCR systems and reagents to
perform PCR amplification.
Single Amplification
In the simplest PCR sequencing case, you use one set of primers to amplify the target
DNA and to sequence the DNA. This method works well for many samples. If your
samples do not work well with this method, you may need to minimize contaminants
to increase the specificity of the PCR amplification and ensure adequate yield (see
page 40).
A single PCR amplification is also compatible with the use of a sequencing primer
which binds internally (semi-nested or nested) to one or both of the PCR primers.
This nested primer approach can be helpful if primer-dimer (primer oligomerization)
artifacts are a problem (see page 240).
Nested and Semi-Nested PCR
If you encounter difficulty with more complex samples, such as bacterial or
eukaryotic genomic DNA, use a nested or semi-nested PCR. Nested and semi-nested
PCR are useful when you have a small quantity of target because these methods
increase specificity. Increased specificity provides superior sequencing data with
reduced background signal. However, these methods may increase the likelihood of
misincorporation because they require two amplifications:
1. Amplify with one set of PCR primers to convert a complex sample (such as
bacterial or eukaryotic genomic DNA) into a non-complex sample consisting of
the first PCR product and some side products.
2. Amplify 1% or less of the first PCR reaction product:
• Nested PCR – Use a second set of PCR primers that hybridize at positions
internal to the first set.
• Semi-nested PCR – Use a second set of PCR primers with one primer that
hybridizes internal to the first set and the other primer that is one of the
original PCR primers.
Universal-Tailed PCR Primers
You can synthesize a universal-tailed PCR primer, which has a universal sequencing
primer binding site added to the 5´ end (see page 263 in Appendix A for universal
pr
ime
r sequences). Uses for universal-tailed PCR primers:
• You can use this technique with dye terminator chemistries because universal
sequencing primers have good annealing characteristics. However, longer PCR
primers increase the reaction cost.

38 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
• You can use commercially available dye-labeled sequencing primers.
• You can use universal sequencing primers to sequence the resulting PCR
product to simplify and standardize the sequencing step.
Fast PCR Versus
Standard PCR
Fast PCR is a new technology that decreases run times by using combination of
protocol changes, fast instruments, and fast reagents. Fast PCR technologies allow
you to:
• Complete PCR runs in less time
• Use less sample and reagents
• Spend more time interpreting results and decision-making
For more information, see the Applied Biosystems application note Advances in Fast
PCR Contribute to a Fast Resequencing Workflow (PN 104AP04-01).
Primer Design and Quantitation
When you perform dye terminator cycle sequencing reactions on PCR template, the
primer sequence, primer synthesis method, and primer purification method can
greatly affect the quality of the sequencing data.
The recommendations in this section are based on general knowledge or on the
practical experience gained by Applied Biosystems scientists.
Optimizing Primer
Design
Recommendations for optimizing primer design:
• Use Applied Biosystems Primer Express
®
Software (PN 4363991) for primer
design:
– To calculate melting temperature (T
m
) accurately
– To identify any secondary hybridization sites on the target DNA
– To identify potential secondary structure problems
• Primers should be at least 18 bases long to ensure good hybridization and to
minimize the probability of hybridizing to a second site on the target DNA.
• Use the recommended thermal cycling conditions for cycle sequencing, because
primers with T
m
>45 °C produce better results than primers with lower T
m
.
• Avoid runs of an identical nucleotide, especially runs of four or more Gs.
• Avoid designing primers over a SNP. Consult SNP databases (dbSNP, SNP500,
and/or SNPbrowser
™
) for SNP locations.
• Keep the G-C content in the range 30 to 80%, preferably 50 to 55%. For primers
with G-C content less than 50%, you may need to increase the primer length
beyond 18 bases to maintain a T
m
>45 °C.
• Avoid primers that can hybridize to form dimers.
• Avoid palindromes because they can form secondary structures.
• The primer should be as pure as possible, preferably purified by HPLC.
• To design primers for sequencing bisulfite-converted DNA, use Applied
Biosystems Methyl Primer Express
®
Software (PN 4376041). The software is
available for free from the Applied Biosystems web site.

39DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Formulas
Calculate the melting temperature, primer concentration, and molecular weight for
your primers.
VariantSEQr
®
Primer Sets
To simplify the identification of sequence variants, Applied Biosystems has pre-
designed 420,000 PCR primer pairs for the resequencing of ~16,000 human genes.
The VariantSEQr
®
primers, which include an M13 sequence tail, are designed for
uniform PCR and sequencing conditions. These primer sequences are available for
free download from the NCBI Probe database www.ncbi.nih.gov/genome/probe.
To download VariantSEQr
®
primer sequences:
1. Go to NCBI’s online probe database: www.ncbi.nlm.nih.gov/genome/probe.
2. Enter “variantseqr and [the symbol for your gene of interest]” in the for field,
then click Go.
3. Check the box next to the probe of interest then click Download to access all
PCR primers for the gene.
4. Select specific primer sets:
• In the graphical representation, click the horizontal lines corresponding to
the primer set of interest.
• In the list of probes, check the checkbox for the primer set of interest.
Alternative transcripts are shown in blue and a union of all transcripts is shown
in black. The RSS ID (Resequencing Set ID) references a set of RSAs
(Resequencing Amplicons) that provide the most efficient coverage. To select
primers for a specific transcript, click the button next to that transcript.
5. Click Download to export the primer sequences. Optionally, check Hide M13
Primers to omit the M13 tail sequences from the exported sequences.
The sequences are saved in a tab-delimited text file (*.txt) on your computer.
Custom Primers
You can obtain custom primers from the Applied Biosystems Custom
Oligonucleotide Synthesis Service.
Feature Formula
Melting temperature (T
m
)T
m
= (number of A + T residues) × 2 °C + (number of G + C residues) × 4°C
Primer concentration
(derived from Beer’s Law)
C (pmol/µL or µM) = (A
260
× 100)/(1.54n
A
+ 0.75n
C
+ 1.17n
G
+ 0.92n
T
)
where:
C = concentration
n
x
= number of residues of base x in the oligonucleotide
Oligonucleotide molecular
weight
Molecular weight of a DNA oligonucleotide (sodium salt, pH 7):
MW = (N
A
x 335.2) + (N
C
x 311.2) + (N
G
x 351.2)+ (N
T
x 326.2) + P
where:
N
X
= number of residues of base x in the oligonucleotide
P = –101.0 for dephosphorylated oligonucleotides, 40.0 for phosphorylated
oligonucleotides

40 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
To order custom primers:
1. Go to Applied Biosystems web site: www.appliedbiosystems.com, register and
log in.
2. Click Products and Services, then Primers and Probes.
3. Click Custom Unlabeled PrimersUnlabeled Oligo.
4. Choose a synthesis scale and click the appropriate Configure button.
5. Select the Formulation option: liquid or dry.
6. Follow the instructions for entering your primer names and sequences or
uploading them from a file.
7. Order primers.
For more information, see How to Order Primers and Probes (PN 113920,
Publication 132GU01-01).
PCR
Contaminants
That Affect Cycle
Sequencing
Products carried over from the PCR amplification can affect cycle sequencing:
• Excess PCR primers – Compete with the sequencing primer for binding sites
and reagents in the sequencing reaction. Additional primers in sequencing
reactions using dye terminators result in the creation of multiple dye-labeled
sequence ladders and noisy data.
• Excess dNTPs – Can affect the dNTP/ddNTP balance of the sequencing
reaction, resulting in a decreased amount of short extension products.
• Nonspecific PCR products – Include primer-dimer artifacts and secondary
PCR products. Nonspecific PCR products behave as templates in the
sequencing reaction and cause the generation of multiple dye-labeled sequence
ladders, which result in noisy data. Any significant quantity of nonspecific PCR
products can result in poor quality sequencing data.
Screen for nonspecific PCR products by running the PCR products on an
agarose gel before sequencing. If you detect nonspecific PCR products,
optimize and repeat the PCR amplification before sequencing. If you use a
nested or semi-nested sequencing primer, you may obtain good sequence data.
Alternatively, you can purify the desired PCR product directly from the agarose
gel as long as the nonspecific PCR product is not the same size as the desired
PCR product. However, significant contamination may remain because of
incomplete separation.
Minimizing
Contaminants
To minimize the contaminants listed above, use the following strategies to increase
the specificity of the PCR amplification:
• PCR optimization strategies (Bartlett and Stirling, 2003; Innis and Gelfand,
1990):
– Amount of starting DNA
– Careful primer design
– Primer concentration
– Enzyme concentration
– Magnesium ion (Mg
2+
) concentration
– Nucleotide concentration
– Buffer composition

41DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
– Number of cycles
–pH
• Manual hot-start method (if the enzyme does not have hot-start capability)
• AmpliTaq Gold
®
DNA Polymerase as an automatic hot start
• Use the following master mixes:
–AmpliTaq Gold
®
360 PCR Master Mix
–AmpliTaq Gold
®
Fast PCR Master Mix , UP (for use with the Veriti
®
96-Well
Fast Thermal Cycler)
Purifying PCR Products for Sequencing
There are several methods for purifying PCR products. Select a method based on the
amounts of components carried over from the PCR reaction and on the sequencing
chemistry you plan to use:
• Ultrafiltration
• Ethanol precipitation
• Gel purification
• Enzymatic purification
IMPORTANT! If more than one PCR product is present, column purification, ethanol
precipitation, or enzymatic purification will not isolate the desired product. Use gel
purification to isolate the desired product or reoptimize the PCR to obtain a single
product. Ultrafiltration may work if the contaminating PCR products are much
smaller than the desired PCR product.
Commercial
Products
Table 5 contains many commercial products that you can use to prepare PCR DNA
templates. This is not an exhaustive list. Applied Biosystems makes no specific
recommendations on the use of these products. Follow the manufacturer’s
instructions to prepare the DNA templates for cycle sequencing.
Table 5 Commercial products for preparing PCR DNA templates
Product Source Description
AMPure
®
Kits PCR
Purification System
Agencourt Bioscience
Corporation
To remove unincorporated dNTPs, primers, and salts by
magnetic separation of nucleic acids.
QuickStep
™
2 PCR
Purification Kits
Edge Biosystems To purify double-stranded DNA using a resin/gel filtration
system in single, 96-well, or 384-well formats.
Montage
®
PCR Filter Units Millipore To remove primers and unincorporated dNTPs from PCR
reactions. Recommended for PCR fragments > 300 bp.
PSIΨClone PCR ST Single
Tube Kit
Princeton Separations To remove primers, nucleotides, enzymes, and salts from
PCR amplification reactions in single tube format.
GENECLEAN
®
Spin Kit MP Biomedicals To purify DNA fragments from agarose gels; remove
proteins, primers, unincorporated nucleotides, and salts
from enzymatic reactions; and isolate PCR product from
genomic DNA and primers.

42 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Cleaning Up PCR
Reactions With
SAP/Exo I
An alternative to one of the more stringent purification methods listed above is
treatment of PCR products with shrimp alkaline phosphatase (SAP) and
Exonuclease I (Exo I) before sequencing. The SAP/Exo I method degrades
nucleotides and single-stranded DNA (primers) remaining after PCR (Werle et al.,
1994).
IMPORTANT! This method does not remove primer-dimers or non-specific PCR
amplification products.
You may need to dilute the PCR product before sequencing. Determine the dilution
ratio empirically (start with 1:2 and 1:10 dilutions with deionized water).
QIAquick
®
Gel Extraction
Kits
QIAGEN To purify PCR fragments from agarose gels.
For DNA ranging from 70 bp to 10 kbp. Larger fragments
should be extracted with the QIAEX II Gel Extraction Kits.
QIAquick
®
PCR Purification
Kits
QIAGEN To clean up DNA (up to 10 kbp). Removes primers,
nucleotides, enzymes, salts, agarose, and ethidium bromide
from DNA samples in individual spin-column, 8-well, or 96-
well formats.
GenElute
™
Minus EtBr Spin
Columns
Sigma-Aldrich To isolate DNA from agarose gels and remove ethidium
bromide.
GenElute
™
PCR Clean-Up
Kit
Sigma-Aldrich To purify single-stranded or double-stranded PCR
amplification products from the reaction components
(excess primers, nucleotides, DNA polymerase, and salts).
ExoSAP-IT
®
USB PCR cleanup to remove excess primers and unincorporated
nucleotides.
Table 5 Commercial products for preparing PCR DNA templates (continued)
Product Source Description
To clean up PCR reactions with SAP/Exo I:
1. For each sample, combine the following:
• SAP (1 Unit/µL), 2.0 µL
• Exo I (10 Units/µL), 0.2 µL
• Deionized water, 6.0 µL
Note: This method works well using 0.5 units of each enzyme per microliter
of PCR products used. SAP buffer is excluded from this protocol because
the method seems to work equally well with or without it.
2. Add the SAP/Exo I mixture (8.2 μL) to the entire PCR product.
3. Incubate at 37 °C for 1 hour.
4. Incubate at 80 °C for 15 minutes to inactivate the enzymes.

43DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Cleaning Up PCR
Reactions with
ExoSAP-IT
The ExoSAP-IT method removes excess PCR primers and unincorporated dNTPs.
These carryover contaminants can present problems in dye terminator chemistry and
cause noisy sequencing data.
To clean up the PCR reactions with ExoSAP-IT:
1. Before opening the reaction plate, centrifuge it at 100 ✕ g for 1 minute.
2. Add 2.0 μL of ExoSAP-IT to each amplified reaction.
3. Seal the plate with MicroAmp
®
Clear Adhesive Film (PN 4306311).
4. Vortex the plate briefly to mix the contents.
5. Centrifuge the plate at 1600 ✕ g for 30 sec.
6. Program the thermal cycler:
7. Set the reaction volume to 12 μL.
8. Place the reaction plate in the thermal cycler, cover the plate with a
MicroAmp
®
Optical Film Compression Pad (PN 4312639), then start the
run.
9. After the run is complete, centrifuge the plate at 100 ✕ g for 1 minute to
bring the contents to the bottom of the wells.
10. Store the plate according to when you are continuing the protocol:
• Within 12 hours – store at 0 to 4 °C
• After 12 hours – store at −15 to −25 °C for 2 to 3 days
Note: Storage times are suggestions only and have not been rigorously
tested.
11. After storage and before opening the plate, centrifuge the plate at 100 ✕ g or
higher for 1 minute.
Stage Description Temp. °C Time
1 Enzyme incubation 37 30 min
2 Enzyme inactivation 80 15 min
3Hold 4Indefinite
hold

44 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
DNA Template Quality
Poor template quality is the most common cause of sequencing problems. Follow
recommended procedures to prepare templates.
Results characteristic of using poor quality templates:
• Noisy data or peaks under peaks (see page 237)
• No or low signal (see page 207 and page 211)
• Early loss or termination of extension (see page 219)
Contaminants
That Affect
Sequencing
Contaminants in cycle sequencing reactions negatively affect polymerase binding
and amplification or extension. Resulting sequences produce poor quality data with
low signal or high noise. Potential contaminants:
• Proteins
•RNA
• Chromosomal DNA
• Excess PCR primers, dNTPs, enzyme, and buffer components (from a PCR
amplification used to generate the sequencing template)
• Residual salts
• Residual organic chemicals, such as phenol, chloroform, and ethanol
• Residual detergents
• Agarose gel, if DNA was extracted from a gel
Examining DNA
Quality
Use both of the following methods to examine DNA quality:
• Agarose gel electrophoresis – Purified DNA should run as a single band on an
agarose gel. Agarose gels reveal contaminating DNAs and RNAs, but not
proteins.
Note: Uncut plasmid DNA can run as three bands: supercoiled, nicked, and
linear. RNA contamination up to 1 µg can be tolerated, but it affects DNA
quantitation greatly.
• Spectrophotometry – The A
260
/A
280
ratio should be 1.8 to 2.0. Smaller ratios
usually indicate contamination by protein or organic chemicals.
Spectrophotometry can reveal protein contamination, but not DNA or RNA
contamination.
Note: Neither agarose gel electrophoresis nor spectrophotometry can reveal
contaminating salts. Salts can interfere with the sequencing reaction, capillary
electrokinetic injection, or electrophoresis, resulting in noisy data.
Cleaning Up Dirty
Templates
You can sometimes clean up a contaminated template with one of the following
methods:
• Ultrafiltration (Microcon or Centricon filter units) – Most efficient method for
salt removal. See Millipore’s web site (www.millipore.com) for instructions on
how to use the Microcon or Centricon filter units.
• Spin columns – May be used for salt removal. See Table 3 on page 34 for
commercial products.

45DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
• Phenol/chloroform extraction – See Sambrook and Russell (2001) for detailed
instructions.
• Ethanol precipitation – May be used for salt removal.
DNA Template Quantity
DNA template quantitation is critical for successful sequencing reactions. The most
common way to determine DNA quantity is to measure the absorbance (optical
density or O.D.) of a sample at 260 nm in a spectrophotometer.
Measuring UV
Absorbance
One O.D. unit is the amount of a substance, dissolved in 1.0 mL, that gives an
absorbance reading of 1.00 in a spectrophotometer with a 1-cm path length. For DNA
quantitation, the wavelength is assumed to be 260 nm unless stated otherwise. A
260
values can be converted into μg/µL using Beer´s Law:
The following formulas are derived from Beer’s Law (Ausubel et al., 1998):
• Concentration of single-stranded DNA = A
260
X 33 μg/µL.
• Concentration of double-stranded DNA = A
260
X 50 μg/µL.
Note: Absorbance measurements of highly concentrated (O.D.>1.0) or very dilute
(O.D.<0.05) DNA samples can be inaccurate. Dilute or concentrate the DNA as
needed to obtain a reading within the acceptable range.
Other Methods
Applied Biosystems makes no specific recommendations on the use of these
products for DNA quantitation:
• Fluorometric analysis using either Hoechst dye #33258 (Hoefer, Inc., 1993) or
PicoGreen
®
dsDNA quantitation reagent (Molecular Probes)
• Fluorometric analysis using Quant-iT
™
assays and the Qubit
®
fluorometer
(Invitrogen)
• Measure UV-Vis absorbance using the NanoDrop ND-1000 spectrophotometer,
which does not require dilution for many sample types (Nanodrop If you have a
Real-Time PCR instrument, you can use Applied Biosystems TaqMan
®
RNase
P Detection Reagents (PN 4316831) to measure DNA quantity.
Absorbance (260 nm) = sum of extinction coefficient contributions X cuvette pathlength X
concentration

46 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Preparing Templates for Bisulfite Sequencing
DNA Extraction
The purity of the gDNA template is critical for the success of a complete bisulfite
conversion. Proteins bound to the gDNA can interfere with the bisulfite conversion
process, resulting in large sections of non-converted sequence. A proteinase K
incubation step is recommended (Warnecke, 2002).
DNA for bisulfite sequencing can be isolated from various sample types including
blood, cultured cells, and tissue [fresh/frozen and formalin-fixed-paraffin embedded
(FFPE)]. Note that FFPE samples can be difficult to analyze due to variation in DNA
quantity, quality and purity.
Depending on source of DNA, you can use any of the following products to prepare
high-quality template for bisulfite sequencing:
• MELT™ Total Nucleic Acid Isolation System (PN AM1983) – For isolating
nucleic acids from tissue without homogenizing tissue samples. After lysis in
MELT solution, DNA is isolated using magnetic bead-based separation
technology.
• RecoverALL™ Total Nucleic Acid Isolation for FFPE Tissues
(PN AM1975) – Optimized to release a maximal amount of RNA and DNA
fragments of all sizes with typical yields of >50% that of unfixed tissue from the
same sample source.
• NucPrep
®
DNA Isolation Kit (PN 4340274) – For recovery of DNA from plant
and animal tissue.
• BloodPrep
®
DNA Isolation Kit (PN 4340274) – For rapid isolation of DNA
from fresh or frozen blood from any animal species, tissue-cultured cells, or
buccal swabs.
Note: Include controls throughout the workflow to monitor incomplete bisulfite
conversion.
Performing Bisulfite Conversion
The bisulfite conversion protocol for Applied Biosystems methylSEQr
™
Bisulfite
Conversion Kit (PN 4379580 consists of three procedures:
1. “Preparing gDNA” on page 46
2. “Performing Bisulfite Conversion” on page 47
3. “Purifying the Sample” on page 48
Preparing gDNA
1. Prepare your gDNA using standard procedures.
2. Determine the gDNA concentration spectrophotometrically at A260 or by
fluorescence.
47DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Performing
Bisulfite
Conversion
CHEMICAL HAZARD. MethylSeqr
™
Conversion Reagent
causes eye, skin, and respiratory tract irritation. Exposure may cause allergic
reactions.
MethylSeqr
™
Denaturation Buffer causes severe eye, skin, and
respiratory tract burns. Read the MSDS and follow the handling instructions. Wear
appropriate protective eyewear, clothing, and gloves.
To convert gDNA using bisulfite:
1. Combine in a labeled microcentrifuge tube:
•5 μL methylSEQr
™
Denaturation Buffer
• Up to 300 ng of gDNA in 45 μL deionized water
The final volume is 50 μL. Seal the tube and thoroughly mix the contents.
IMPORTANT! Do not exceed 300 ng of gDNA per conversion reaction.
Excess gDNA may produce an incomplete bisulfite conversion.
2. Incubate the tube at 37 °C for at least 15 minutes. Continue holding at 37 °C
while preparing the conversion reagent in step 3.
3. Prepare the methylSEQr
™
Conversion Reagent. To one tube of methylSEQr
Conversion Reagent, add:
•750 μL deionized water
•210 μL methylSEQr
™
Denaturation Buffer
Note: Each prepared tube of methylSEQr Conversion Reagent is enough for
10 samples.
4. Seal the tube and vortex to mix as follows:
• Vortex for 1 minute
• Let rest for 2 minutes
• Repeat 5 times
IMPORTANT! Protect the prepared methylSEQr Conversion Reagent from
light and use within 1 hour of preparation.
5. Remove the denatured gDNA from 37 °C incubation and add 100 μL of the
conversion reagent to each sample. The final volume is 150 μL.
6. Incubate the sample(s) in the dark at 50 °C for 12 to 16 hours.

48 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
Purifying the
Sample
CHEMICAL HAZARD. Sodium hydroxide (NaOH) causes
severe eye, skin, and respiratory tract burns. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
To purify bisulfite-converted samples:
1. Add 200 to 300 μL of deionized water to an assembled methylSEQr
™
Purification Column.
2. Add the 150-μL incubated sample and mix by pipetting up and down.
Be careful not to puncture the column membrane.
3. Centrifuge the sample at a maximum of 500 ✕ g for 20 minutes, then discard
the filtrate.
4. Add 350 μL of deionized water to the upper chamber of the purification
column filter.
5. Centrifuge the column at 500 ✕ g for 20 minutes, then discard the filtrate.
6. Repeat steps 4 and 5.
7. Add 350 μL of 0.1 M sodium hydroxide to the upper chamber of the
purification column filter and allow the mixture to sit for 5 minutes.
8. Centrifuge the column at 500 ✕ g for 20 minutes, then discard the filtrate.
9. Add 350 μL of deionized water to the upper chamber, then centrifuge the
column at a maximum of 500 ✕ g for 15 to 20 minutes, until the membrane
is just damp, or a small amount of liquid remains in the upper chamber.
10. Add 50 μL of 1✕ TE buffer to the upper chamber and mix gently by
pipetting up and down. Let the column stand for 5 minutes.

49DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation
11. Invert the column as shown and collect the bisulfite-treated gDNA in a clean
microcentrifuge tube by centrifuging at 1000 ✕ g for 30 seconds.
The sample is now ready for PCR.
12. Store any unused bisulfite-converted gDNA at 4 °C. The bisulfite-converted
gDNA can be used in PCR for up to one year as long as there is no
contamination.
Note: Do not subject the bisulfite-converted gDNA to freeze-thawing.
To purify bisulfite-converted samples: (continued)

50 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 3 DNA Template Preparation

53DNA Sequencing by Capillary Electrophoresis Chemistry Guide
4
Cycle Sequencing 4
This chapter covers:
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .54
Choosing a Sequencing Chemistry . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .55
Reagent and Equipment Considerations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .61
DNA Quantity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .63
Using DNA Template Controls . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .64
Using BigDye Terminators and dGTP BigDye Terminators. . . . . . . . . . . . . . . . . . .66
Using dRhodamine Terminators . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .72
Using BigDye Primers. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .73
Direct Sequencing From Cultures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .75
Bisulfite Sequencing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .77

54 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Overview
This chapter provides information on how to select the sequencing chemistry and
cycle sequencing conditions for each chemistry.
Workflow
DNA Template Preparation (Chapter 3)
Cycle Sequencing
1. Select the sequencing chemistry.
2. Prepare cycle sequencing reactions.
3. Run sequencing reactions in a thermal
cycler.
Applied Biosystems Cycle Sequencing
Kits:
• BigDye Terminator v1.1 and v3.1 kits
• dGTP BigDye Terminator v1.0 and v3.0
kits
• BigDye Primer kits
• dRhodamine Terminator kits
Cycle Sequencing Output:
Extension Product Purification (Chapter 5)
Capillary Electrophoresis (Chapter 6)
Data Analysis (Chapter 7)
F1 F2 F3 F4
1 2 3
4 5 6
7 8 9
ENTER
STOP
0 CE
F5
GeneAmp
®
PCR System 9700
POWER
G
e
n
e
A
m
p
®
P
C
R
S
y
s
t
e
m
9
7
0
0
9700 thermal
cycler
9800 thermal
cycler
Veriti
®
thermal
cycler
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
A
C
C
G
T
A
A
C
C
G
T
A
C
C
G
T
A
C
C
G
A
C
C
A
CAA
T
or
Dye terminator products
Dye primer products

55DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Choosing a Sequencing Chemistry
Selection Criteria
When choosing a sequencing chemistry, consider the following:
• DNA sequencing application
• Sequence context
• DNA template type
• Length of desired read
Available Kits
Use Table 6 and Table 7 to select the cycle sequencing kit that meets your needs:
Table 6 Applied Biosystems cycle sequencing kits
Kit Description Page
BigDye
®
Terminator v3.1 Cycle
Sequencing Kits
• Compatible with all automated genetic analyzers on Table 1 on page 10
• For comparative sequencing; de novo sequencing; resequencing; and
sequencing PCR products, plasmids, cosmids, fosmids, and large
templates, for example, bacterial artificial chromosome (BAC) clones
• Can sequence difficult templates and dinucleotide repeats
• Formulated with dITP in place of dGTP to reduce peak compressions
57
BigDye
®
Terminator v1.1 Cycle
Sequencing Kits
• Compatible with automated genetic analyzers on Table 1 on page 10
• For optimal basecalling adjacent to the primer and sequencing short
PCR product templates
‡
• Can sequence difficult templates and dinucleotide repeats
• Formulated with dITP in place of dGTP to reduce peak compressions
57
dGTP BigDye
®
Terminator
Cycle Sequencing Kits
• Compatible with automated genetic analyzers on Table 1 on page 10
• Formulated with dGTP to produce higher-quality data when sequencing
G-related motifs such as GT-, GA-, and GC-rich templates
58
dRhodamine Terminator Cycle
Sequencing Kits
• Compatible with 3100/3100-Avant Genetic Analyzer and 310 Genetic
Analyzer
• For sequencing templates with homopolymers (AA, CC, GG, or TT) or T-
rich motifs
• Formulated with dITP in place of dGTP to reduce peak compressions
59
BigDye
®
Primer Cycle
Sequencing Kits
• Compatible with 3100/3100-Avant Genetic Analyzer and 310 Genetic
Analyzer
• For sequencing single- and double-stranded DNA and PCR templates
• Generates data with uniform peak heights and optimal basecalling
adjacent to the primer
60
‡ In sequences obtained using BigDye Terminators v1.1 when run on rapid run modules and with a fast run polymer (POP-
7 and POP-4), base mobility in beginning sequences is slightly worse than in sequences obtained using BigDye
Terminators v3.1. However, when using BigDye Terminators v1.1, peak resolution of small fragments is better with POP-
6 polymer.

56 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Table 7 shows recommended chemistries, based on the sequencing application and/or
characteristics of the DNA to be sequenced.
Ratings are:
• Recommended: ++
• Satisfactory: +
• Not recommended: –
Table 7 Chemistry recommendations for different sequencing applications and/or template
characteristics
Application
BigDye
Term in a t or
v3.1
BigDye
Term in a t or
v1.1
dGTP
BigDye
Term in a t or
dRhodamine
Term in a t or
BigDye
Primer
DNA Sequencing Application
Bisulfite sequencing + ++ - – –
Comparative sequencing
(germline mutations 50:50 heterozygotes)
++ ++ – + ++
Comparative sequencing
(somatic mutations 10:90 heterozygotes)
––– –+
Comparative sequencing
(somatic mutations 30:70 heterozygotes)
++– –++
De novo sequencing ++ ++ + + +
Gap closure (custom primers) ++ ++ ++
‡
+–
Gene walking (custom primers) ++ ++ + + –
Shotgun sequencing (universal primers, M13) ++ ++ + + ++
DNA Sequence Context
AT-rich >65% ++ ++ – ++ ++
GC-rich >65% ++ ++ + + +
GT-rich regions + + ++ ++ ++
Homopolymer A or T >25 bp
§
––– ++–
Te m p l a t e Ty p e
BAC, cosmid, fosmid, lambda, large PCR
product
++ ++ – + +
Bacterial genomic DNA ++ ++ – – –
Bisulfite-treated genomic DNA + ++ – – –
PCR amplicon ++ ++ + ++ ++
PCR amplicon (heterozygous 10:90) – – – – +

57DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
BigDye
Te rmin ato r
v1.1/3.1 Cycle
Sequencing Kits
With BigDye Terminator v1.1 and v3.1 kits, you can sequence difficult templates and
read through dinucleotide repeats and other challenging sequence motifs, using a
wide range of template types and qualities. You can use these kits for a wide range of
sequencing applications and obtain peak-height uniformity and optimized signal
balance for longer, higher quality reads.
Note the peak uniformity and high quality basecalls across the GT repeats in
Figure 26.
Figure 26 Peak uniformity and high quality basecalls using BigDye terminators
These kits provide the required reagent components for the sequencing reaction in a
ready reaction, pre-mixed format. You need only to provide your template and the
template-specific primer.
Note: These kits include BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5✕),
which has been specifically optimized for use with the v1.1 and v3.1 BigDye
®
Ready
Reaction Mixes.
For more information, refer to the BigDye
®
Terminator v3.1 Cycle Sequencing Kit
Protocol (PN 4337035) or the BigDye
®
Terminator v1.1 Cycle Sequencing Kit
Protocol (PN 4337036).
PCR amplicon (heterozygous 30:70) + + – – ++
PCR amplicon (heterozygous 50:50) ++ ++ – + ++
Plasmid (<15 kb) ++ ++ + ++ ++
Rolling circle amplified product ++ ++ + ++ ++
Single-stranded DNA ++ ++ + ++ ++
‡ Recommended for sequencing gaps with difficult GT- and GA-rich motifs.
§ All cycle sequencing chemistries can have difficulties with homopolymers >40 bp.
Table 7 Chemistry recommendations for different sequencing applications and/or template
characteristics (continued)
Application
BigDye
Term in a t or
v3.1
BigDye
Term in a t or
v1.1
dGTP
BigDye
Term in a t or
dRhodamine
Term in a t or
BigDye
Primer

58 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
dGTP BigDye
Terminator Cycle
Sequencing Kits
The dGTP BigDye
®
Terminator v1.0/v3.0 Cycle Sequencing Kits were developed for
use with difficult templates where the standard terminator kits give data with early
signal loss.
These kits use dGTP in the deoxynucleoside triphosphate mix instead of the dITP
used in standard Applied Biosystems dye terminator cycle sequencing kits. The dITP
is used in dye terminator kits to minimize peak compressions, but the substitution
can lead to early signal loss in some sequence contexts.
The electropherogram in Figure 27 shows the sequence of a region of a plasmid that
did not give satisfactory sequence results with other chemistry kits. Sequencing
using dGTP BigDye terminators resulted in high quality basecalls through the
difficult-to-sequence GT-rich region.
Figure 27 Sequence through a GT-rich region sequenced using dGTP BigDye
terminators
IMPORTANT!
Because of compressions, Applied Biosystems does not recommend
using the dGTP BigDye Terminator v1.0/v3.0 Cycle Sequencing Kits for routine
sequencing. They should be used only when you cannot obtain good data using
standard terminator kits.
For more information, refer to:
• dGTP BigDye
®
Terminator Ready Reaction Kit Protocol (PN 4307169)
or
• dGTP BigDye
®
Terminator v3.0 Ready Reaction Cycle Sequencing Kit Protocol
(PN 4390038).

59DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
dRhodamine
Terminator Cycle
Sequencing Kits
dRhodamine Terminator Cycle Sequencing Kits can be used to perform
fluorescence-based cycle sequencing reactions on single-stranded or double-stranded
DNA templates, or on polymerase chain reaction (PCR) fragments. The dRhodamine
kit is effective for sequencing through homopolymer regions and T-rich motifs.
The dRhodamine dyes have a narrow emission spectra, with small spectral overlap
and therefore low noise (Figure 7 on page 8).
Figure 28 shows sequence through two homopolymer T regions in a plasmid
sequenced using dRhodamine terminators. Note the minimal amount of slippage
after each homopolymer region using dRhodamine terminators.
Figure 28 Sequence through two homopolymer T regions in a plasmid
sequenced using dRhodamine terminators
These kits provide the required reagent components for the sequencing reaction in a
ready reaction, pre-mixed format. You need only provide your template and the
template-specific primer.
For more information, refer to the dRhodamine Terminator Cycle Sequencing Ready
Reaction Kit Protocol (PN 403041).

60 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
BigDye Primer
Cycle
Sequencing Kits
BigDye Primer Cycle Sequencing Kits can be used to perform fluorescence-based
cycle sequencing reactions on single-stranded or double-stranded DNA templates, on
polymerase chain reaction (PCR) fragments, and on large templates, such as the ends
of BAC clones. With these kits:
• The A, C, G, and T reactions are carried out in separate tubes or wells and in a
1:1:1:1 ratio.
• The deoxynucleotide/dideoxynucleotide mixes have been optimized to provide
reads above 700 bases.
• Formulations contain 7-deaza-dGTP in place of dGTP to minimize peak
compressions.
• Large templates, such as the ends of BAC clones, can be sequenced readily.
In Figure 29, note the peak uniformity throughout the general sequence using
BigDye primers.
Figure 29 Peak uniformity throughout the general sequence using BigDye
primers
In the Ready Reaction format, the dye-labeled primers, deoxynucleoside
triphosphates, dideoxynucleoside triphosphates, DNA polymerase, pyrophosphatase,
magnesium chloride, and buffer are premixed into A, C, G, and T Ready Reaction
cocktails to eliminate time-consuming reagent preparation.
For more information, refer to the BigDye
®
Primer Cycle Sequencing Ready
Reaction Kit Protocol (PN 403057).

61DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Reagent and Equipment Considerations
Reagent Handling
and
Reaction Storage
For optimal performance using Applied Biosystems sequencing chemistry reagents,
follow these recommendations. Handle and store other reagents according to
manufacturer’s recommendations.
• Store reagents in uncolored tubes or vials at –15 to –25 °C when not in use, and
thaw completely at room temperature or in an ice bath (do not heat) before use.
Note: Do not use a frost-free freezer. The automatic cycling of the temperature
for defrosting can damage reagents, particularly enzymes.
• Avoid excessive freeze-thaw cycles. Aliquot reagents in smaller amounts if
necessary.
• Protect reagents and sequencing reactions from light. Fluorescent dyes are
susceptible to bleaching.
• To store sequencing reactions for future use, purify the extension products, then
dry the reactions. Store the purified, dried reactions at –15 to –25 °C.
Note: Do not store sequencing reactions at −70 to −80 °C.
• Sequencing reactions purified with the BigDye XTerminator Purification Kit
can be stored as sealed reaction plates for up to 48 hours at room temperature or
up to 10 days at 4 °C or −20 °C without having to dry down the reactions.
Preventing Dye
Degradation
All fluorescent dyes are sensitive to degradation by a variety of chemical and
physical agents. Degradation of the dyes can affect the sequencing analysis results
(pages 231 though 234).
To prevent dye degradation:
• Protect fluorescently labeled DNA from light, heat, acidic conditions, and
oxygen.
IMPORTANT! Use the BigDye XTerminator Purification Kit to purify samples
after cycle sequencing. After purification, samples are stable for up to 48 hours
at room temperature or up to 10 days at 4 °C or −20 °C.
•Use fresh Hi-Di
™
Formamide. Old Hi-Di Formamide or low quality formamide
will contain formic acid, which can contribute to the degradation of fluorescent
dyes.
IMPORTANT! If the Hi-Di Formamide does not solidify at −20 °C, then it
should be discarded.
IMPORTANT! If the sequencing reactions are purified with the BigDye
XTerminator Purification Kit, do not add Hi-Di Formamide.
• Heat-seal plates for the 3730/3730xl instruments if you are preparing multiple
plates.
• After resuspending samples, run them on the instrument as quickly as possible.

62 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Reaction Tubes
and Plates
Use tubes or plates appropriate for the thermal cycler:
• GeneAmp
®
PCR Systems 9700 or Veriti
®
96-Well Thermal Cycler
–0.2-mL MicroAmp
®
Reaction Tubes (PN N8010533)
or
–MicroAmp
®
Optical 96-Well Reaction Plate (PN N8010560)
• Applied Biosystems 9800 Fast PCR System or Veriti
®
FAST 96-Well Thermal
Cycler
– 96-Well Fast Thermal Cycling Plate (PN 4346907)
Note: Fast plates require a 96-Well Fast (0.1mL) Plate Base (PN 4367470) and
a 96-Well Fast (0.1mL) Plate Retainer (PN 4367471) for sequencing. For more
information, see the Introducing New 96-Well Fast Plate Adapters for Applied
Biosystems Capillary Electrophoresis Systems User Bulletin (PN 4370890).
For high-throughput demands, use:
• MicroAmp Optical 96-Well Reaction Plates with Barcode (PN 4306737)
or
• MicroAmp Optical 384-Well Reaction Plates with Barcode (PN 4309849)
Other plates and tubes may be used. Refer to the user guide for your thermal cycler
for part numbers.
Thermal Cyclers
The thermal cycling conditions in this chemistry guide were optimized using the
GeneAmp PCR System 9700, the Applied Biosystems 9800 Fast PCR System, and
the Veriti
®
96-Well Thermal Cyclers. If you choose to use a thermal cycler not
manufactured by Applied Biosystems, you may need to adjust the thermal cycling
conditions due to differences in ramp rates and thermal accuracy. The ramp rate for
thermal cyclers not manufactured by Applied Biosystems should be 1 °C/second.
The type and performance of the thermal cycler can affect the quality of the
reactions. Make sure that the thermal cycler is calibrated as recommended by the
manufacturer.

63DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
DNA Quantity
DNA Template
Quantities
The amount of DNA template used in a sequencing reaction can affect the quality of
the data. Too much template makes data appear top heavy, with strong peaks at the
beginning of the run that fade rapidly. Too little template or primer reduces the signal
strength and peak height. In the worst case, the noise level increases so that bases
cannot be called.
DNA sequencing reactions purified with the BigDye
®
XTerminator™ Purification
Kit result in high signal strength when analyzed on a DNA sequencer. When you
prepare sequencing samples for purification with the BigDye XTerminator reagents,
you may need to decrease the amount of DNA template in the sequencing reactions
to keep the fluorescence signals on scale during analysis.
Table 8 shows the recommended quantities of DNA template for each sequencing
chemistry and for samples purified with the BigDye XTerminator Purification Kit.
Note: For information about preparing DNA templates for sequencing, see
Chapter 3.
Table 8 Recommended DNA template quantities for cycle sequencing
Tem pla te
Cycle Sequencing Chemistry
BigDye
Te r mi na to r
v1.1 and v3.1
BigDye
XTerminator
Purification Kit
dGTP BigDye
Te r mi n a t o r
v3.0
dRhodamine
Term in a t or
BigDye Primer
PCR product:
100 to 200 bp 1 to 3 ng 0.5 to 3 ng 1 to 3 ng 1 to 3 ng 2 to 5 ng
200 to 500 bp 3 to 10 ng 1 to 10 ng 3 to 10 ng 3 to 10 ng 5 to 10 ng
500 to 1000 bp 5 to 20 ng 2 to 20 ng 5 to 20 ng 5 to 20 ng 10 to 20 ng
1000 to 2000 bp 10 to 40 ng 5 to 40 ng 10 to 40 ng 10 to 40 ng 20 to 50 ng
>2000 bp 40 to 100 ng 10 to 50 ng 40 to 100 ng 40 to 100 ng 50 to 150 ng
Bisulfite converted
genomic DNA-PCR
product
3 to 10 ng 3 to10 ng not
recommended
not
recommended
not
recommended
Single-stranded DNA 50 to 100 ng 10 to 50 ng 50 to 100 ng 50 to 100 ng 150 to 400 ng
Double-stranded DNA 200 to 500 ng 50 to 300 ng 200 to 500 ng 200 to 500 ng 200 to 800 ng
Cosmid, BAC 0.5 to 1.0 µg 200 to 1,000 ng 0.5 to 1.0 μgnot
recommended
0.5 to 1.0 µg
Bacterial genomic
DNA
2 to 3 µg 1,000 to 3,000 ng 2 to 3 μgnot
recommended
not
recommended

64 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Using DNA Template Controls
Recommended
Controls
Include a DNA template control in each set of sequencing reactions. The results from
this control can help you determine whether failed reactions are caused by poor
template quality or sequencing reaction failure.
Applied Biosystems recommends M13mp18 as a single-stranded control and
pGEM
®
-3Zf(+) as a double-stranded control. Applied Biosystems DNA sequencing
kits provide pGEM control DNA at 0.2 μg/μL. Applied Biosystems dye terminator
cycle sequencing kits include a –21 M13 control primer at 0.8 pmol/μL. For control
sequences, see page 263.
Sequencing
Control
Reactions
Sequencing Control Reactions for Samples Prepared with BigDye XTerminator
Purification Kit
Reagent
Quantity Per Reaction (μL)
96-Well 384-Well
‡
‡Performing 10-μL reactions in 384-well reaction plates allows you to perform the post-
reaction cleanup step in the same well.
Ready Reaction Mix 8.0 4.0
pGEM − 3Z(+) Control Template (0.2 μg/μL) 1.0 0.5
− 21 M13 forward primer (0.8 pmol/μL) 4.0 2.0
Deionized water 7.0 3.5
Total Volume 20.0
10.0
Reagent
Quantity Per Reaction (μL)
96-Well
(20μL reaction)
96-Well
(10μL reaction)
384-Well
(5μL reaction)
‡
‡Performing 10-μL reactions in 384-well reaction plates allows you to perform the post-
reaction cleanup step in the same well.
Ready Reaction Mix 8.0 4.0 2.0
pGEM − 3Z(+) Control Template
(0.2 μg/μL)
0.5 0.25 0.125
− 21 M13 forward primer
(0.8 pmol/μL)
4.0 2.0 1.0
Deionized water 7.5 3.75 1.875
Total Volume 20.0
10.0 5.0

65DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Thermal Cycling
Conditions
Table 9 Thermal cycling conditions using DNA template controls
Mode Thermal Cycling Conditions
• 9600 emulation
mode
• 9700 standard
mode
• 9800 standard
mode
•Veriti
®
96-Well
Thermal Cycler
• 9800 Fast
mode
•Veriti
®
FAST
96-Well
Thermal Cycler
Stage Description Temp. (°C) Time
1 Denaturation 96 1 min
2 Amplification: 25 cycles 96 10 sec
50 5 sec
60 4 min
3 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 96 1 min
2 Amplification: 25 cycles 96 10 sec
50 5 sec
60 75 sec
3 Hold 4 Indefinite hold

66 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Using BigDye Terminators and dGTP BigDye Terminators
Sequencing
Reaction
Components
.
Using BigDye
Te rmina to r
v1.1/v3.1
Sequencing
Buffer
Ready Reaction Mix contains BigDye Sequencing buffer and other components. If
you are using less of the Ready Reaction Mix, add 5✕ BigDye Terminator
Sequencing Buffer to bring the final buffer concentration to 1✕. The chemistry is
optimized for a full strength reaction. Different instruments have different
sensitivities and different detection systems.
Depending on the instrument you use, template quantity, and desired read length, you
can make modifications using the following calculation.
For a given volume of Ready Reaction Mix:
IMPORTANT! BigDye Terminator v1.1/v3.1 Sequencing Buffer is intended for use
only with BigDye Terminator v1.1/v3.1 Cycle Sequencing Kits.
Note: The use of the BigDye Terminator v1.1/v3.1 Sequencing Buffer without
optimization may result in deterioration of sequencing quality.
Reagent
Quantity Per Reaction
96-Well
(20μL reaction)
96-Well or 384-Well
(10μL reaction)
‡
‡ Performing 10-μL reactions in 384-well reaction plates allows you to perform post-reaction
cleanup in the same well.
384-Well
(5μL reaction)
Ready Reaction Mix 8.0 μL4.0μL2.0μL
Template Quantity depends on template type and size.
§
§See Table 8, “Recommended DNA template quantities for cycle sequencing,” on page 63.
Primer 3.2 pmol 3.2 pmol 3.2 pmol
Deionized water q.s. q.s. q.s.
Total Volume 20 μL
10 μL
‡
5 μL
0.5 (8 μL- Ready Reaction Mix Volume) = Volume of BigDye Terminator Sequencing Buffer
to add

67DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Thermal Cycling
Conditions
The thermal cycling conditions in Table 10 work for a variety of templates and
primers. These thermal cycling conditions were optimized using the GeneAmp PCR
System 9700, the Applied Biosystems 9800 Fast Thermal Cycler, the Veriti 96-Well
Thermal Cycler, and the Veriti FAST 96-Well Thermal Cycler. If you choose to use
other thermal cyclers, you may need to adjust the conditions because of differences
in ramp rates and thermal accuracy.
Table 10 Thermal cycling conditions for BigDye terminators and dGTP BigDye terminators
DNA Template Thermal Cycling Conditions
BigDye Terminators v1.1 and v3.1 (9600 Emulation Mode, 9700 Standard Mode, 9800 Standard Mode, and Veriti
96-Well Thermal Cycler)
•Double-stranded DNA
•Single-stranded DNA
•PCR product
BAC DNA
Stage Description Temp. (°C) Time
1 Denaturation 96 1 min
2 Amplification: 25 cycles 96 10 sec
50 5 sec
60 4 min
3 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 95 5 min
2 Amplification: 50 cycles
‡
‡ Some laboratories have found that increasing the number of cycles gives
better results.
95 30 sec
50 to 55
§
§ Set the annealing temperature according to the template.
10 sec
60 4 min
3 Hold 4 Indefinite hold

68 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Bacterial genomic DNA
Bisulfite-treated template-
PCR amplicon
Table 10 Thermal cycling conditions for BigDye terminators and dGTP BigDye terminators (continued)
DNA Template Thermal Cycling Conditions
Stage Description Temp. (°C) Time
1 Denaturation 95 5 min
2 Amplification: 45 cycles 95 30 sec
50 to 55
‡
‡ Set the annealing temperature according to the template.
20 sec
60 4 min
3 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 96 10 min
2 Amplification: 25 cycles 96 10 sec
50 4 min
3 Hold 4 Indefinite hold

69DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
dGTP BigDye Terminators v1.0 (9600 Emulation Mode, 9700 Standard Mode, 9800 Standard Mode, and Veriti
96-Well Thermal Cycler)
•Double-stranded DNA
•Single-stranded DNA
•PCR product
dGTP BigDye Terminators v3.0 (9600 Emulation Mode, 9700 Standard Mode, 9800 Standard Mode, and Veriti
96-Well Thermal Cycler)
•Double-stranded DNA
•Single-stranded DNA
•PCR product
Table 10 Thermal cycling conditions for BigDye terminators and dGTP BigDye terminators (continued)
DNA Template Thermal Cycling Conditions
Stage Description Temp. (°C) Time
1 Amplification: 25 cycles 96 10 sec
50 10 sec
60 4 min
2 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Amplification: 25 cycles 96 10 sec
68 2 min
2 Hold 4 Indefinite hold

70 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Modifying
Thermal Cycling
Conditions
You may modify the thermal cycling conditions. Applied Biosystems makes the
following suggestions for modifying these conditions. These suggestions have not
been exhaustively tested at Applied Biosystems and they have not been found to
work for all cases:
• For short PCR products, try reducing the extension time (for example, 2 minutes
for a 300 bp or smaller fragment instead of 4 minutes) or reducing the number
of cycles from 25 to 20.
Note: For sequencing DNA with difficult contexts, decreasing extension times
may result in reduced quality in the length of read and signal strength.
• If you observe high background signal and the T
m
of a primer is >60 °C, try
eliminating the annealing step.
• If you observe low signals and the T
m
of a primer is <50 °C, increase the
annealing time to 30 seconds or decrease the annealing temperature to 48 °C.
BigDye Terminators v1.1/v3.1 and dGTP BigDye Terminators v1.0/v3.0 (9800 Fast Mode and Veriti FAST 96-Well
Thermal Cycler)
•Double-stranded DNA
•Single-stranded DNA
•PCR product
BigDye Terminators v3.1 (9800 Fast Mode and Veriti FAST 96-Well Thermal Cycler)
BAC DNA
Table 10 Thermal cycling conditions for BigDye terminators and dGTP BigDye terminators (continued)
DNA Template Thermal Cycling Conditions
Stage Description Temp. (°C) Time
1 Denaturation 96 1 min
2 Amplification: 25 cycles 96 10 sec
50 5 sec
60 75 sec
3 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 96 2 min
2 Amplification: 50 cycles 96 20 sec
50 10 sec
60 2 min
3 Hold 4 Indefinite hold

71DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
• For templates with high GC content (>70%), heat the tubes at 98 °C for
5 minutes before cycling to help denature the template.
• For sequencing large templates such as BACs and fosmids, increasing the
number of cycles may help increase signal.

72 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Using dRhodamine Terminators
Sequencing
Reaction
Components
Thermal Cycling
Conditions
Reagent
Quantity Per Reaction
96-Well 384-Well
Ready Reaction Mix 8.0 μL4.0μL
Template: Quantity depends on template size.
‡
‡See Table 8, “Recommended DNA template quantities for cycle sequencing,”
on page 63.
single-stranded DNA
double-stranded DNA
PCR product DNA
50 to 100 ng
200 to 500 ng
1 to 100 ng
50 to 100 ng
200 to 500 ng
1 to 100 ng
Primer 3.2 pmol 3.2 pmol
Deionized water q.s. q.s.
Total Volume 20 μL
10 μL
§
§Performing 10-μL reactions in 384-well reaction plates allows you to perform the
post-reaction cleanup step in the same well.
Table 11 Thermal cycling conditions using dRhodamine terminators
Mode Thermal Cycling Conditions
• 9600 emulation
mode
• 9700 standard
mode
• 9800 standard
mode
• Veriti 96-Well
Thermal Cycler
• 9800 Fast
mode
•Veriti FAST
96-Well
Thermal Cycler
Stage Description Temp. (°C) Time
1 Amplification: 25 cycles 96 10 sec
50 5 sec
60 4 min
2 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 96 1 min
2 Amplification: 25
cycles
96 10 sec
50 5 sec
60 75 sec
3 Hold 4 Indefinite hold

73DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Using BigDye Primers
The cycle sequencing protocols in this section are for using BigDye Primers in the
9600 emulation mode and the 9700 standard mode. Applied Biosystems has not
validated cycle sequencing protocols for using BigDye Primers in 9800 Fast mode or
for the Veriti thermal cyclers. If you want to use 9800 Fast mode or the Veriti thermal
cycler, you may need to experiment to determine an effective protocol.
When sequencing using BigDye primers, select the reaction type and cycle type
according to the DNA template:
IMPORTANT! Prepare separate tubes for each of the four reactions (A, C, G, and T).
1X Reaction
Components
High-Sensitivity
(2X) Reaction
Components
Template Reaction Type
Cycle
Type
•PCR product
•plasmid
•M13
1X standard
Large DNA templates:
•Genomic DNA
• Bacterial artificial chromosomes (BACs)
• Yeast artificial chromosomes (YACs)
•Cosmids
• Long PCR products containing M13 forward and/or
M13 reverse priming site(s)
High-sensitivity (2X) modified
Reagent
Quantity Per Reaction (μL)
ACGT
Ready Reaction Premix 4.0 4.0 4.0 4.0
DNA template (see Ta b l e 8 o n
page 63 for quantity)
1.0 1.0 1.0 1.0
Total Volume 5.0 5.0 5.0 5.0
Reagent
Quantity Per Reaction (μL)
ACGT
Ready Reaction Premix 8.0 8.0 8.0 8.0
DNA template (see Ta b l e 8 o n
page 63 for quantity)
2.0 2.0 2.0 2.0
Total Volume 10.0 10.0 10.0 10.0

74 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Thermal Cycling
Conditions
The thermal cycling conditions are for 9600 emulation mode and 9700 standard
mode. Select the conditions appropriate for the reaction type.
Table 12 Thermal cycling conditions using BigDye primers
Reaction Type Thermal Cycling Conditions
1✕
High-sensitivity
(2✕)
Stage Description Temp. (°C) Time
1 Amplification: 15 cycles 96 10 sec
55 5 sec
70 1 min
2 Amplification: 15 cycles 96 10 sec
70 1 min
3 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1 Denaturation 95 5 min
2 Amplification: 20 cycles 95 30 sec
50 15 sec
70 1 min
3 Amplification: 15 cycles 95 30 sec
70 1 min
4 Hold 4 Indefinite hold

75DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Direct Sequencing From Cultures
Direct sequencing from cultures bypasses plasmid template preparation, reducing
costs and work and allowing for faster screening of libraries for sequences of interest.
However, previous protocols for direct sequencing produce a lower success rate and
shorter read lengths. The protocols in this section can be used with BigDye
®
Terminator v1.1 and v3.1 Cycle Sequencing Kits to produce success rates and read
lengths comparable to high throughput purified plasmid sequencing. The protocols
are optimized for direct sequencing from cultures or colonies for various template
types, in 96-well (below) or 384-well formats (page 76).
Note: For purification of samples that are directly sequenced from a colony, use
ethanol precipitation.
96-Well Direct
Sequencing
To perform direct sequencing from cultures (96-well):
1. Pick a colony or transfer glycerol backup to 200 μL TB/Amp (Costar 96-
well cell culture cluster #3585) and incubate for 20 hours (no shaking).
2. Prepare cell lysate:
a. Transfer 50 μL of the culture to a 96-well reaction plate.
b. Centrifuge the plate at 4000 rpm for 10 minutes. Remove the
supernatant by inverting the plate, then centrifuge the plate again until
the speed reaches 500 rpm.
c. Resuspend the cell pellets in 12 μL of water by vortexing, then
centrifuge the plate at 1000 rpm for 1 minute.
d. Heat the cell lysate at 95 °C for 3 minutes, then cool it to 4 °C.
3. Set up cycle sequencing reactions:
Reagent
Volume
(μL)
Cell lysate 4.0
Sequencing primer (3.2 μM) 1.0
BigDye Terminator v3.1 4.0
Deionized water 1.0
Tota l
10.0

76 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
384-Well Direct
Sequencing
4. Perform cycle sequencing on the 9700 thermal cycler (9600 emulation
mode):
5. Precipitate the DNA, performing the ethanol precipitation protocol for
96-well, 10-μL reaction volumes (page 97).
To perform direct sequencing from cultures (96-well): (continued)
Stage Description Temp. (°C) Time
1 Denaturation 96 10 sec
2 Amplification: 50 cycles 50 5 sec
3604 min
4 Hold 4 Indefinite hold
To perform direct sequencing from cultures (384-well):
1. Pick a colony or transfer glycerol backup to 50 μL TB/Amp (Nalgene 384-
well plate, cell culture treated #164610) and incubate for 20 hours (no
shaking).
2. Prepare cell lysate:
a. Transfer 5 μL of the culture to a 384-well reaction plate.
b. Centrifuge the plate at 4000 rpm for 10 minutes. Remove the
supernatant by inverting the plate, then centrifuge at 1000 rpm for
1 minute.
c. Resuspend cell pellets in 2 μL of water by vortexing, then centrifuge
the plate at 1000 rpm for 1 minute.
d. Heat the cell lysate at 95 °C for 3 minutes, then cool to 4 °C.
3. Set up cycle sequencing reactions:
Reagent
Volume
(μL)
Cell lysate 2.0
Sequencing primer (3.2 μM) 1.0
BigDye Terminator v3.1 2.0
To t a l
5.0

77DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Bisulfite Sequencing
PCR
Amplification
Bisulfite-converted DNA can be a difficult template to amplify. After bisulfite
conversion of gDNA, the double stranded nucleic acid is transformed into single
stranded template, comprised of 5 different bases: A, G, T, U, and 5mC. Methylated
promoter regions tend to be 5mCpG rich. C-G base pair-rich islands are known to be
difficult to amplify.
Cloning
Amplification bias, slippage, and low primer specificity can make direct sequencing
of PCR products difficult. Sequencing clones, rather than direct sequencing of
amplicons derived from bisulfite-converted DNA template, can provide clean
sequence, because clone sequencing produces a single amplicon insert per clone.
Although sequencing clones can be time consuming, advantages include:
• No secondary sequence
• No PCR slippage
• No mixed bases
• Elimination of misaligned sequences due to mobility differences
• All 4 bases are represented for signal normalization due to sequence content
from the cloning vector
• Ability to provide a semi-quantitative percentage of methylation
Bisulfite cloning followed by sequencing permits the assessment of methylation
haplotypes for individual samples. Analyzing many clones can provide an estimate of
the percentage of methylated targets at an individual site. However, like all
quantitative methylation strategies, cloning is subject to biases, which limit the
accuracy of quantitation.
4. Perform cycle sequencing on the 9700 thermal cycler (9600 emulation
mode):
5. Precipitate the DNA, performing the ethanol precipitation protocol for
384-well, 10-μL volumes (page 99).
To perform direct sequencing from cultures (384-well): (continued)
Stage Description Temp. (°C) Time
1 Denaturation 96 10 sec
2 Amplification: 50 cycles 50 5 sec
3604 min
4 Hold 4 Indefinite hold

78 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Sources of bias can include:
• Purity of the sample extracted from biological sources
• Completeness of the bisulfite conversion
• Degree of bias in the bisulfite conversion purification step
• Number of clones sequenced
Polymerase Selection
After bisulfite conversion of gDNA, the double stranded nucleic acid is transformed
into single stranded template, composed of 5 different bases: A, G, T, U, and 5mC.
The polymerase used for PCR amplification must be capable of reading U and 5mC
during the first round of synthesis in the reverse compliment strand. Master mixes
containing uracil DNA glycosylase (UNG) should not be used, because the UNG
cleaves U-containing DNA, degrading the template. High fidelity polymerase from
archaebacteria such as Vent or Pfu DNA polymerase should not be used because they
are strongly inhibited by uracil.
Use a hot-start polymerase in conjunction with relatively high temperature to avoid
mismatch and amplification.
PCR Bias
The template derived from the unmethylated strand is frequently amplified more
efficiently than the template derived from the methylated strand. The template
derived from the unmethylated strand therefore dominates in a mixed sample.
Tips for reducing PCR bias when amplifying templates of mixed methylated states
include:
• Use a hot-start PCR enzyme such as AmpliTaq Gold
®
DNA Polymerase or a
PCR master mix containing this enzyme
• Use tailed primers with all 4 bases in design
• Use a PCR denaturant such as glycerol
• Set the annealing temperature approximately 2 to 5 °C above the calculated T
m
(gene-specific portion)
• Increase the annealing temperature after the first few PCR cycles
• Perform Touchdown PCR
• Increase the extension time/temperature during PCR
• Decrease primer concentration (to reduce primer-dimer formation)

79DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
Cycle
Sequencing for
Bisulfite-Treated
gDNA
The protocol below is optimized for bisulfite PCR using M13-tailed primers with
a 5 °C
T
m
for the gene-specific portion of the primer. For fragment analysis, a
dye-label (FAM) is included on the
5′ end of one of the primers. The tailed primer
sequences are:
•-21 M13 Forward Primer – 5′ TGTAAAACGACGGCCAGT 3′
• M13 Reverse Primer – 5′ CAGGAAACAGCTATGACC 3′
To cycle sequence bisulfite-treated gDNA using M13 primers:
1. Prepare a solution containing 5 mg/mL BSA and 5% glycerol:
\
2. Set up cycle sequencing reactions. For each 5 μL reaction:
Reagent
Volume
(μL)
GeneAmp
®
10✕ Gold Buffer & MgCl
2
250
dNTP 2.5 mM each 700
Water 50
Total 1000
Reagent
Volume
(μL)
GeneAmp
®
10✕ Gold Buffer & MgCl
2
0.5
dNTP 2.5 mM each 0.4
MgCl
2
25mM 0.4
AmpliTaq Gold
®
DNA polymerase (5U/μL) 0.1
Forward primer (5μM) 0.25
Reverse primer (5μM) 0.25
Bisulfite gDNA template (6ng/μL) 0.5
BSA-glycerol solution (from step 1 on page 79 0.5
Water 2.1
Tota l 5.0

80 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 4 Cycle Sequencing
3. If your sample is only for sequencing, perform cycle sequencing on the
9700 thermal cycler (9600 emulation mode) as follows:
If you plan to perform fragment analysis, use the following conditions to
perform cycle sequencing on the 9700 thermal cycler (9600 emulation
mode):
Note: The additional 60 minutes at 60 °C at the end of thermal cycling
allows full conversion to the non-template A-addition amplicon product.
To cycle sequence bisulfite-treated gDNA using M13 primers: (continued)
Stage Description Temp. (°C) Time
1Activate 95 5 min
2 Amplification: 40 cycles 95 30 sec
3602 min
4723 min
5 Hold 4 Indefinite hold
Stage Description Temp. (°C) Time
1Activate 95 5 min
2 Amplification: 5 cycles 95 30 sec
3602 min
4723 min
5 Amplification: 35 cycles 95 30 sec
6651 min
7723 min
8Hold 60 60
9 Hold 4 Indefinite hold

Part Number 4305080 Rev. C 05/2009

85DNA Sequencing by Capillary Electrophoresis Chemistry Guide
5
Purification of Extension Products 5
This chapter covers:
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .86
Purification with the BigDye® XTerminator™ Purification Kit . . . . . . . . . . . . . . .88
Purification by Ethanol Precipitation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .96
Purification with Spin Columns and Spin Plates . . . . . . . . . . . . . . . . . . . . . . . . . .116
Alternative Purification Procedures . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .119
Sample Preparation for Electrophoresis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .120

86 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Overview
This chapter presents procedures for purifying extension products and
recommendations for preparing the purified samples for electrophoresis.
Workflow
Why Purification
is Needed
The presence of both unlabeled and dye-labeled reaction components can interfere
with electrokinetic injection, electrophoretic separation, and data analysis. For
instance, fluorescent signals from unincorporated dye-labeled terminators that co-
migrate with sequencing reaction extension products obscure the desired signal and
interfere with basecalling. Purification of extension products can reduce or eliminate
this interference. Methods for purifying extension products vary according to user
preference and the cycle sequencing chemistry employed.
Dye Terminator
Chemistries
Applied Biosystems supplies three dye terminator chemistries:
•BigDye
®
Terminators v1.1 and 3.1
• dGTP BigDye
®
Terminators v1.0 and v3.0
• dRhodamine Terminators
DNA Template Preparation (Chapter 3)
Cycle Sequencing (Chapter 4)
Extension Product Purification
Purify extension products using one method:
•BigDye
®
XTerminator
™
Purification
• Ethanol precipitation
• Spin-column purification
• Alternative cleanup procedures
After purification, prepare samples for electrophoresis
Extension Product Purification Output:
Purified dye terminator products or purified dye primer products
Capillary Electrophoresis (Chapter 6)
Data Analysis (Chapter 7)

87DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
For each sequencing chemistry, there are different methods for removing excess dye
terminators, using different reagents and processes. Applied Biosystems
recommends performing controlled reactions with each method to determine the one
that works best for you.
• Ethanol precipitation methods tend to be less expensive than commercially
available products designed for this purpose, but these methods tend to be labor
intensive and they are prone to variation in performance, depending upon a
variety of factors. For more information, refer to the Precipitation Methods to
Remove Residual Dye Terminators from Sequencing Reactions User Bulletin
(PN 4304655).
• Methods using commercially available products such as the BigDye
®
XTerminator Purification Kit, spin columns, size-exclusion membranes, and
magnetic beads efficiently remove terminators if performed correctly. Many of
the commercial methods may be adapted to laboratory robotic systems. See
page 119 for a list of currently available commercial products.
Dye Primer
Chemistries
For dye primer chemistries (BigDye
®
Primers v3.0), the standard method for
purifying extension products is ethanol precipitation, which concentrates the sample.

88 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Purification with the BigDye
®
XTerminator
™
Purification Kit
Overview
The BigDye
®
XTerminator
™
Purification Kit sequesters cycle-sequencing reaction
components such as salt ions, unincorporated dye terminators, and dNTPs, to prevent
their co-injection with dye-labeled extension products into a capillary electrophoresis
DNA analyzer.
The BigDye XTerminator reagents are compatible with BigDye
®
Terminators v1.1
and v3.1. Purification of cycle sequencing products with other dye chemistries has
not been tested.
Samples purified with BigDye XTerminator can be directly injected into the
capillary electrophoresis using BigDye XTerminator-specific run modules. The run
modules are designed for the 3730/3730xl 3130/3130xl, and 3100/3100–Avant
Analyzers, with Data Collection Software v2.0 or later.
To run samples purified with BigDye XTerminator on other instrument
configurations, the purified sample must be transferred to a new plate.
DNA sequencing reactions purified with the BigDye XTerminator Purification Kit
result in high signal strength. Follow the quantity guidelines in Table 8,
“Recommended DNA template quantities for cycle sequencing,” on page 63.
You can purify samples using two types of pipetting:
• Premix pipetting – Prepare a mixture of the two BigDye XTerminator reagents
(referred to here as “premix”) then pipette the premix into the reaction plate (see
“Preparing the XTerminator Premix” and “Performing Purification Using the
Premix” starting on page 90).
• Sequential pipetting – Add SAM
™
Solution to the reaction plate first, followed
by the XTerminator Solution (see “Performing Purification Using Sequential
Pipetting” on page 93).
For more information see the BigDye
®
XTerminator
™
Purification Kit Protocol
(PN 4374408).
Advantages of
Using the
BigDye
®
XTerminator
™
Purification Kit
• Simplifies workflow:
– No need for spin column or ethanol precipitation
–Fast
– Little hands-on time needed
• Flexible throughput with purification in single tubes, 96-well or 384-well
formats
• No bead removal prior to injection (depending upon the instrument used for
sequencing)
• Removes dye “blobs” efficiently
• No danger of removing pellet (as can happen with ethanol precipitation)
• Higher signal intensity for the purified sample decreases quantity of DNA
required
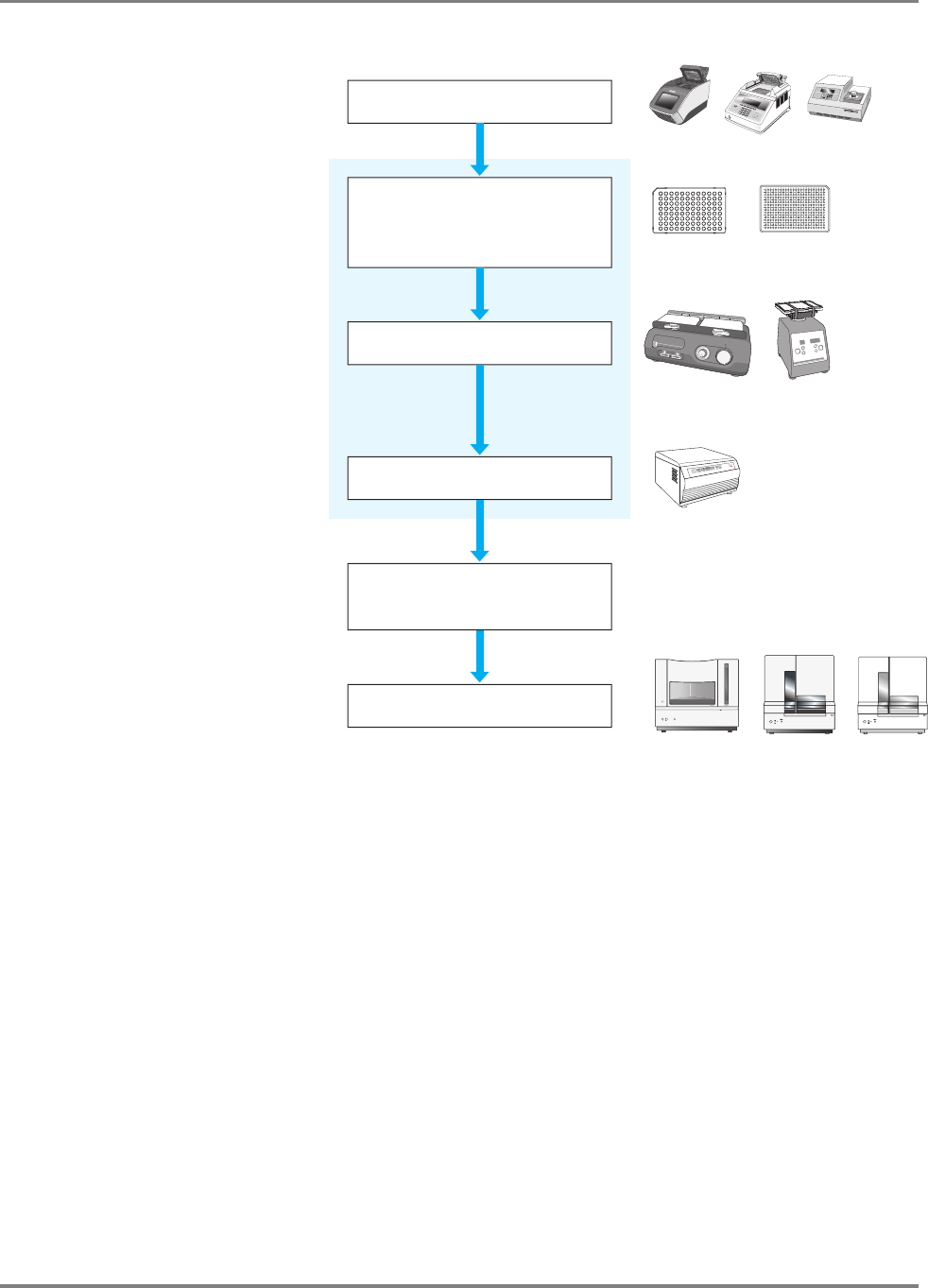
89DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
BigDye
®
XTerminator
™
Purification Kit
Workflow
Figure 30 Sequencing workflow with extension product purification using the
BigDye XTerminator Purification Kit
Important Tips for
Using the BigDye
XTerminator
Purification Kit
• When you pipette directly from the XTerminator Solution bottle:
– Before pipetting, mix the XTerminator Solution until homogeneous.
– Use wide-bore pipette tips.
– Avoid pipetting near the surface of the liquid.
– When you seal the reaction plate, verify that each well is sealed.
• To achieve optimum performance, use a recommended vortexer and follow the
protocol when you vortex the reaction plate.
Prepare premix and
add BigDye
®
XTerminator™
reagents to reaction plate
Vortex reaction plate
Perform cycle sequencing
Extension Product Purification
96
0
0
G
e
n
o
t
y
p
in
g
PC
R
S
y
s
t
e
m
G
eneAm
p
®
PC
R System
9700
A
B
C
D
E
F
G
H
123456789101112
384-well plate96-well plate
Thermal cyclers
G R 2170
M ic r o F lu id ic C a r d
Sorvall C entrifuge
Centrifuge
3730/3130/3100 instruments
Vor texer
Centrifuge plate
Select appropriate run module
in Data Collection software
Perform electrophoresis

90 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Preparing the
XTerminator
Premix
Guidelines for Preparing the Premix
These guidelines apply to single- and multi-dispense pipettes.
• Use wide-bore pipette tips (tips with an orifice >1.0 mm) for pipetting the
XTerminator Solution.
• Use conventional pipette tips for pipetting the SAM Solution.
• Agitate the XTerminator Solution for at least 10 seconds using a standard
laboratory vortexer at maximum speed before pipetting.
IMPORTANT! XTerminator Solution that is allowed to stand for more than 2
minutes must be revortexed.
Note: If refrigerated, the premix is stable for no more than 5 days. Make only the
volume of premix that you will use in 5 days.
Note: You may see fewer reactions per kit when using the premix method.
CHEMICAL HAZARD. SAM Solution is a flammable liquid
and vapor. It may be harmful if absorbed through the skin, inhaled or swallowed.
Exposure may cause eye, skin, and respiratory tract irritation, liver damage, and
central nervous system depression. Read the MSDS, and follow the handling
instructions. Wear appropriate protective eyewear, clothing, and gloves.
To prepare BigDye XTerminator premix:
1. Based on your plate and reaction size, calculate the volumes of XTerminator
Solution and SAM Solution needed.
Note: All volumes below include an additional 10% to account for dead
volume.
Plate Type and
Reaction
Volume/Well
Volume/Well (μL) Volume/Plate (μL)
XTerminator
™
Solution
SAM
™
Solution
XTerminator
™
Solution
SAM
™
Solution
384-well, 5 μL 5.5 24.75 2112 9504
96-well, 10 μL 11 49.5 1056 4752
96-well, 20 μL 22 99 2112 9504

91DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Performing
Purification Using
the Premix
Guidelines for Pipetting the Premix
• Use conventional pipette tips when pipetting the premix.
• When pipetting from a bottle, keep the premix agitated using a rocking motion.
IMPORTANT! Using a stir bar on a stir plate does not keep the premix properly
suspended.
• When pipetting from a trough, keep the premix agitated by:
– Rocking the trough back and forth lengthwise to create a wave motion.
or
– Placing the pipette tips 1 to 2 mm above the trough bottom and moving them
gently from side-to-side.
• Agitate the XTerminator premix before each aspiration.
2. Combine the SAM Solution and the XTerminator Solution to create the
premix:
a. Vortex the XTerminator Solution bulk container at maximum speed for
at least 10 seconds, until it is homogeneous.
b. Using a wide-bore pipette tip or a graduated cylinder, transfer the
appropriate volume of XTerminator Solution to a clean container.
IMPORTANT! Insert the pipette tip well below the surface of the liquid
before aspirating.
c. Using a conventional pipette tip or graduated cylinder, add the
appropriate volume of SAM Solution to the container with the
XTerminator Solution.
Make sure that there are no particulates in the SAM Solution before
pipetting. If particulates are present, heat the SAM Solution to 37 °C
and mix to redissolve. Cool to room temperature before using.
d. Mix the reagents until homogenous.
3. Either store the premix up to 5 days for later use or continue to “Performing
Purification Using the Premix” on page 91.
To prepare BigDye XTerminator premix: (continued)
To purify samples with BigDye XTerminator premix:
1. Be sure the premix is well mixed, then transfer it to the trough or reservoir.
2. After cycle sequencing is complete, centrifuge the reaction plate or tube,
for 1 minute.

92 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
3. Into each well of the reaction plate, use a conventional pipette tip to add the
volume of premix specified below:
Add more premix to the trough or reservoir as necessary.
IMPORTANT! For 384-well reactions with reaction volume less than 5 mL,
add water to bring the volume to 5 mL before adding SAM Solution. For
96-well reactions with reaction volume less than 10 mL, add water to bring
the volume to 10 mL before adding SAM Solution.
IMPORTANT! Dispense the premix within 1 minute of aspiration to avoid
separation of the reagents in the pipette tip.
4. Seal the plate using:
• A heat seal at 160 °C for 1.5 seconds.
or
• MicroAmp
®
Clear Adhesive Films.
Verify that each well is sealed.
IMPORTANT! If you use a 3730/3730xl instrument and plan to use direct
injection without a septa mat, only Applied Biosystems Heat Seal Film for
Sequencing and Fragment Analysis Sample Plates (PN 4337570) is
supported.
To purify samples with BigDye XTerminator premix: (continued)
Plate Type and Reaction
Volume/Well
Volume of Premix/Well
(μL)
384-well, 5-μL 27.5
96-well, 10-μL55
96-well, 20-μL 110

93DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Performing
Purification Using
Sequential
Pipetting
CHEMICAL HAZARD. SAM Solution is a flammable liquid
and vapor. It may be harmful if absorbed through the skin, inhaled or swallowed.
Exposure may cause eye, skin, and respiratory tract irritation, liver damage, and
central nervous system depression. Read the MSDS, and follow the handling
instructions. Wear appropriate protective eyewear, clothing, and gloves.
5.
Vortex the reaction plate for 30 minutes, using the following conditions:
Note: Pause vortexing after 1 minute and examine the wells to verify that
the contents are well mixed.
6. In a swinging-bucket centrifuge, spin the plate at 1000 ✕ g for 2 minutes.
7. Proceed with “Sample Preparation for Electrophoresis” on page 120.
To purify samples with BigDye XTerminator premix: (continued)
Vortexer Plate Type Speed
Digital Vortex-Genie 2 96-well 1800 rpm
384-well 2000 rpm
Eppendorf MixMate 384-well 2600 rpm
IKA MS3 Digital Either 2000 rpm
‡
‡ Set the vortexer to Mode B. See the BigDye
®
XTerminator
™
Purification Kit
Protocol for instructions.
IKA Vortex 3 Either Setting 5
§
§ Use the maximum setting that does not cause the vortexer to “walk” across the
bench.
Taitec MicroMixer E-36 Either Maximum
Union Scientific Vertical Shaker
#
# Add any additional plates to meet mass requirements. See the BigDye
®
XTerminator
™
Purification Kit Protocol for information.
Either Setting 100
To purify samples using sequential pipetting:
1. After cycle sequencing is complete, centrifuge the reaction plate for
1 minute to spin down the plate contents.

94 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
2. To each well of the reaction plate, add the volume of SAM Solution
specified below using a conventional pipette tip.
Make sure there are no particulates in the SAM Solution before pipetting. If
particulates are present, heat the SAM Solution to 37 °C and mix to
resuspend. Cool to room temperature before using.
IMPORTANT! For 384-well reactions with reaction volume less than 5 μL,
add water to bring the volume to 5 μL before adding SAM Solution. For 96-
well reactions with reaction volume less than 10 μL, add water to bring the
volume to 10 μL before adding SAM Solution.
3. Add the XTerminator Solution:
a. Vortex the XTerminator Solution bulk container at maximum speed for
at least 10 seconds, until it is homogeneous.
b. Use a wide-bore pipette tip to aspirate the XTerminator Solution.
IMPORTANT! Avoid pipetting from the top of the liquid.
c. Into each well, add the volume of XTerminator Solution specified
below:
d. Discard the pipette tip.
4. Seal the plate using:
• A heat seal at 160 °C for 1.5 seconds.
or
• MicroAmp
®
Clear Adhesive Films.
Verify that each well is sealed.
IMPORTANT! If you use a 3730/3730xl instrument and plan to use direct
injection without a septa mat, only Applied Biosystems Heat Seal Film for
Sequencing and Fragment Analysis Sample Plates (PN 4337570) is
supported.
To purify samples using sequential pipetting: (continued)
Plate Type and Reaction
Volume/Well
Volume of SAM
™
Solution/Well (μL)
384-well, 5-μL22.5
96-well, 10-μL45
96-well, 20-μL90
Plate Type and Reaction
Volume/Well
Volume of XTerminator
™
Solution/Well (μL)
384-well, 5-μL5
96-well, 10-μL10
96-well, 20-μL20

95DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
5.
Vortex the reaction plate for 30 minutes, using the following conditions:
It is recommended to pause vortexing after 1 minute and examine the wells
to verify that the contents are well mixed.
6. In a swinging-bucket centrifuge, spin the plate at 1000 ✕ g for 2 minutes.
7. Proceed with “Sample Preparation for Electrophoresis” on page 120.
To purify samples using sequential pipetting: (continued)
Vortexer Plate Type Speed
Digital Vortex-Genie 2 96-well 1800 rpm
384-well 2000 rpm
Eppendorf MixMate 384-well 2600 rpm
IKA MS3 Digital Either 2000 rpm
‡
‡ Set the vortexer to Mode B. See the BigDye
®
XTerminator
™
Purification Kit
Protocol for instructions.
IKA Vortex 3 Either Setting 5
§
§ Use the maximum setting that does not cause the vortexer to “walk” across the
bench.
Taitec MicroMixer E-36 Either Maximum
Union Scientific Vertical Shaker
#
# Add any additional plates to meet mass requirements. See the BigDye
®
XTerminator
™
Purification Kit Protocol for information.
Either Setting 100

96 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Purification by Ethanol Precipitation
Alcohol-based nucleic acid precipitation techniques include a wide variety of
methods. Variations include: choice of alcohol (isopropanol, butanol or ethanol),
choice and concentration of salt (NaOAc, NaCl, NH
4
OAc, KCl or LiCl), temperature
(–20 to 0 °C) and additives such as EDTA and Mg
2+
. These variations are designed to
yield slight differences in the precipitation products. Users are encouraged to
experiment to obtain the specific technique best suited to their needs. The protocols
below have been shown to obtain good performance across a broad variety of sample
types.
Ethanol
Concentrations
Inaccurate final ethanol concentrations can affect sequencing results. If ethanol
concentrations are too low, you will not precipitate all of the extension products and
you will lose them when you remove the supernatant. If ethanol concentrations are
too high, you will precipitate unincorporated terminators with the extension
products, producing large peaks (blobs) in the electropherogram (page 230).
• You may use 95% ethanol, but you must make sure to maintain the same final
ethanol concentration for precipitation (65 to 71%).
• The ethanol concentration in absolute ethanol decreases gradually because
absolute ethanol absorbs water from the atmosphere. Replace ethanol stocks
frequently.
Methods
Select a method appropriate for the dye chemistry you use:
•BigDye
®
Terminators v1.1 and v3.1
– Ethanol/EDTA precipitation: page 96
– Ethanol/EDTA/sodium acetate precipitation: page 101
• dGTP BigDye
®
Terminators v1.0 and v3.0
– Ethanol/sodium acetate precipitation: page 105
– Ethanol precipitation: page 105
• dRhodamine Terminators
– Ethanol/sodium acetate precipitation: page 109
– Shrimp alkaline phosphatase digestion (optional): page 110
– Simplified ethanol precipitation: page 110
•BigDye
®
Primers: page 114
Ethanol Precipitation for BigDye
®
Terminators v1.1 and v3.1
Purification
Methods
• Ethanol/EDTA precipitation:
– Precipitating in 96-well reaction plates (below)
– Precipitating in 384-well reaction plates (page 99)
• Ethanol/EDTA/Sodium acetate precipitation:
– Precipitating in 96-well reaction plates (page 101)
– Precipitating in 384-well reaction plates (page 103)

97DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol/EDTA
Precipitation
With the BigDye terminators v1.1 and v3.1, the ethanol/EDTA precipitation method
produces consistent signal and is particularly good for removing unincorporated dye-
labeled terminators.
Note: This method produces the cleanest signal, but it may cause loss of small
fragments.
CHEMICAL HAZARD. EDTA. Exposure causes eye irritation.
Read the MSDS, and follow the handling instructions. Wear appropriate protective
eyewear, clothing, and gloves.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
To precipitate 10-μL or 20-μL reactions in 96-well reaction plates:
1. Remove the 96-well reaction plate from the thermal cycler and centrifuge
the plate at 100 ✕ g for 1 minute, then remove the seal.
2. Add the appropriate amount of 125 mM EDTA, pH 8.0 to each well.
• For 10 μL reactions – 2.5 μL
• For 20 μL reactions – 5 μL
IMPORTANT! Make sure the EDTA reaches the bottom of the wells.
3. Add the appropriate amount of ethanol to each well so that the final ethanol
concentration is 67 to 71%.
4. Seal the plate securely with self-adhesive film, then invert the plate 4 times
to mix the contents.
or
Mix the contents well by pipetting up and down in a multichannel pipette 3
to 4 times, then seal the plate securely with self-adhesive film.
Sequencing
Reaction Volume
Amount of
100% Ethanol to Add
Amount of
95% Ethanol to Add
10 μL30μL36μL
20 μL60μL72μL

98 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
5. Incubate at room temperature for 15 minutes, then centrifuge the plate:
Note: Using a centrifuge at 4 °C will yield better recovery of smaller
molecular weight fragments, although a room temperature centrifuge can be
used.
IMPORTANT! Proceed to the next step immediately. If this is not possible,
continue centrifuging the plate until you are ready to perform the next step.
6. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing the spin when the rotor starts moving.
7. Add the appropriate amount of 70% ethanol to each well.
• For 10 μL reactions – 30 μL
• For 20 μL reactions – 60 μL
8. Seal the plate with self-adhesive film, then centrifuge the plate at 1650 ✕ g
for 15 minutes.
9. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge up to 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing when the rotor starts moving.
10. Make sure the wells are dry. You can use a vacuum centrifuge for 5 minutes
to dry the plate.
IMPORTANT! Protect the samples from light while they are drying.
IMPORTANT! Do not heat the samples to speed up ethanol evaporation.
11. (Optional) If you plan to store the plate before proceeding with
electrophoresis, seal the plate tightly with aluminum tape and store it at
−20 °C protected from light.
12. Proceed with “Minimum Sample Volume” on page 122.
To precipitate 10-μL or 20-μL reactions in 96-well reaction plates: (continued)
If you are using... Then...
a Beckman Allegra 6A
centrifuge with a GH-3.8A
rotor
set the centrifuge to 4 °C and centrifuge the
plate at 1650 ✕ g for 45 minutes
any other centrifuge use a plate adapter and centrifuge the plate
at the maximum speed at 4 °C as follows:
• 1400 to 2000 ✕ g for 45 minutes
or
• 2000 to 3000 ✕ g for 30 minutes

99DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Precipitating in 384-Well Reaction Plates
Note:
When precipitating sequencing reactions less than 10 μL, add enough
deionized water to the reactions to bring the volume to 10 μL. Then follow the
protocol below for precipitation.
CHEMICAL HAZARD. EDTA. Exposure causes eye irritation.
Read the MSDS, and follow the handling instructions. Wear appropriate protective
eyewear, clothing, and gloves.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves
To precipitate 10-μL sequencing reactions in 384-well reaction plates:
1. Remove the 384-well reaction plate from the thermal cycler and centrifuge
the plate at 100 ✕ g for 1 minute, then remove the seal.
2. Add 2.5 μL of 125 mM EDTA, pH 8.0 to each well.
IMPORTANT! Make sure the EDTA reaches the bottom of the wells.
3. Add 25 μL of 100% ethanol to each well.
Note: The final concentration of ethanol should be 67 to 71%. The use of
95% ethanol is not recommended because the final volume would exceed
38 μL (the maximum final volume when using 384-well plates).
4. Seal the plate securely with self-adhesive film, then invert the plate 4 times
to mix the contents.
or
Mix the contents well by pipetting up and down in a multichannel pipette 3
to 4 times, then seal the plate securely with self-adhesive film.

100 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
5. Incubate the plate at room temperature for 15 minutes, then centrifuge the
plate:
Note: If you use a room temperature centrifuge, you may not get complete
recovery of small fragments.
IMPORTANT! Proceed to the next step immediately. If this is not possible,
then spin the tubes for an additional 2 minutes immediately before
performing the next step.
6. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing the spin when the rotor starts moving.
7. Add 30 μL of 70% ethanol to each well, seal the plate with self-adhesive
film, then centrifuge the plate at 1600 to 2000 ✕ gg for 15 minutes.
8. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing the spin when the rotor starts moving.
9. Make sure the wells are dry. You can use a vacuum centrifuge for 5 minutes
to dry the plate.
IMPORTANT! Protect the samples from light while they are drying.
IMPORTANT! Do not heat the samples to speed up ethanol evaporation.
10. (Optional) If you plan to store the plate before proceeding with
electrophoresis, seal the plate tightly with aluminum tape and store it at
−20 °C protected from light.
11. Proceed with “Minimum Sample Volume” on page 122.
To precipitate 10-μL sequencing reactions in 384-well reaction plates: (continued)
If you are using... Then...
a Beckman Allegra 6A
centrifuge with a GH-3.8A
rotor
set the centrifuge to 4 °C and centrifuge the
plate at 1650 ✕ g for 45 minutes
any other centrifuge use a plate adapter and centrifuge the plate
at the maximum speed at 4 °C as follows:
• 1400 to 2000 ✕ g for 45 minutes
or
• 2000 to 3000 ✕ g for 30 minutes

101DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol/EDTA/
Sodium Acetate
Precipitation
Ethanol/EDTA/sodium acetate precipitation is recommended when you require good
signal for sequences close to the end of the primer. However, for reactions containing
high concentrations of unincorporated terminators, some residual terminators may be
carried through the precipitation. To completely remove excess terminators in these
cases, ethanol/EDTA precipitation is recommended (see page 97).
CHEMICAL HAZARD. EDTA. Exposure causes eye irritation.
Read the MSDS, and follow the handling instructions. Wear appropriate protective
eyewear, clothing, and gloves.
CHEMICAL HAZARD. 3 M sodium acetate may cause eye,
skin, and respiratory tract irritation. Read the MSDS, and follow the handling
instructions. Wear appropriate protective eyewear, clothing, and gloves.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
To precipitate sequencing reactions in 96-well reaction plates:
1. Remove the 96-well reaction plate from the thermal cycler and centrifuge
the plate at 100 ✕ g for 1 minute, then remove the seal.
2. Add the appropriate amount of 125 mM EDTA, pH 8.0 to each well.
• For 10 μL reactions – 1 μL
• For 20 μL reactions – 2 μL
IMPORTANT! Make sure the EDTA reaches the bottom of the wells.
3. Add the appropriate amount of 3 M sodium acetate to each well.
• For 10 μL reactions – 1 μL
• For 20 μL reactions – 2 μL
Note: Make sure the sodium acetate reaches the bottom of the wells.
4. Add the appropriate amount of ethanol to each well.
Sequencing
Reaction Volume
Amount of
100% Ethanol to Add
Amount of
95% Ethanol to Add
10 μL25μL30μL
20 μL50μL60μL

102 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
5. Seal the plate securely with self-adhesive film, then mix by inverting the
plate 4 times.
or
Mix well by pipetting up and down in a multichannel pipette 3 to 4 times,
then seal the plate securely with self-adhesive film.
6. Incubate the plate at room temperature for 15 minutes, then centrifuge the
plate:
IMPORTANT! Proceed to the next step immediately. If this is not possible,
then spin the tubes for an additional 2 minutes immediately before
performing the next step.
7. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing the spin when the rotor starts moving.
8. Add the appropriate amount of 70% ethanol to each well.
9. Seal the plate with self-adhesive film, then with the centrifuge set to 4 °C,
spin the plate at 1650 ✕ g for 15 minutes.
10. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge up to 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing when the rotor starts moving.
To precipitate sequencing reactions in 96-well reaction plates: (continued)
If you are using... Then...
a Beckman Allegra 6A
centrifuge with a GH-3.8A
rotor
set the centrifuge to 4 °C and centrifuge the
plate at 1650 ✕ g for 45 minutes
any other centrifuge use a plate adapter and centrifuge the plate
at the maximum speed at 4 °C as follows:
• 1400 to 2000 ✕ g for 45 minutes
or
• 2000 to 3000 ✕ g for 30 minutes
Sequencing
Reaction Volume
Amount of
70% Ethanol
to Add
10 μL35μL
20 μL70μL

103DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Precipitating in 384-Well Reaction Plates
When precipitating sequencing reactions that are less than 10 μL, add enough
deionized water to the reactions to bring the volume up to 10
μL. Then follow the
protocol below for precipitation.
CHEMICAL HAZARD. EDTA. Exposure causes eye
irritation. Read the MSDS, and follow the handling instructions. Wear appropriate
protective eyewear, clothing, and gloves.
CHEMICAL HAZARD. 3 M sodium acetate may cause eye,
skin, and respiratory tract irritation. Read the MSDS, and follow the handling
instructions. Wear appropriate protective eyewear, clothing, and gloves.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
11. Make sure the wells are dry. You may use a vacuum centrifuge for 5 minutes
to dry the plate.
IMPORTANT! Protect the samples from light while they are drying.
IMPORTANT! Do not heat the samples to speed up ethanol evaporation.
12. (Optional) If you plan to store the plate before proceeding with
electrophoresis, seal the plate tightly with aluminum tape and store it at
−20 °C, protected from light.
13. Proceed with “Minimum Sample Volume” on page 122.
To precipitate 10-μL sequencing reactions in 384-well reaction plates:
1. Remove the 384-well reaction plate from the thermal cycler, centrifuge the
plate at 100 ✕ g for 1 minute, then remove the seal.
2. Add 1
μL of 125 mM EDTA, pH 8.0 to each well.
IMPORTANT! Make sure that the EDTA reaches the bottom of the wells.
3. Add 1
μL of 3 M sodium acetate to each well.
Note: Make sure that the sodium acetate reaches the bottom of the wells.
To precipitate sequencing reactions in 96-well reaction plates: (continued)

104 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
4. Add 25 μL of 100% ethanol to each well.
Note: The final volume in the 384-well plate should not exceed 38 μL.
Note: The final ethanol concentration should be 67 to 71%.
Note: Use of 95% ethanol is not recommended because the final volume
would exceed 38
μL.
5. Seal the plate securely with self-adhesive film, then mix by inverting the
plate 4 times.
or
Mix well by pipetting up and down in a multichannel pipette 3 to 4 times,
then seal the plate securely with self-adhesive film.
6. Incubate at room temperature for 15 minutes and then centrifuge the plate:
IMPORTANT! Proceed to the next step immediately. If this is not possible,
then spin the tubes for an additional 2 minutes immediately before
performing the next step.
7. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
8. Add 30 μL of 70% ethanol to each well, seal the plate with self-adhesive
9. Remove the self-adhesive film, invert the plate onto a paper towel,
centrifuge at 185 ✕ g for 1 minute, then remove the plate from the
centrifuge.
Note: Start timing when the rotor starts moving.
10. Make sure the wells are dry. You may use a vacuum centrifuge for 5 minutes
to dry the plate.
IMPORTANT! Protect the samples from light while they are drying.
IMPORTANT! Do not heat the samples to speed up ethanol evaporation.
To precipitate 10-μL sequencing reactions in 384-well reaction plates: (continued)
If you are using... Then...
a Beckman Allegra 6A
centrifuge with a GH-3.8A
rotor
set the centrifuge to 4 °C and centrifuge the
plate at 1650 ✕ g for 45 minutes
any other centrifuge use a plate adapter and centrifuge the plate
at the maximum speed at 4 °C as follows:
• 1400 to 2000 ✕ g for 45 minutes
or
• 2000 to 3000 ✕ g for 30 minutes

105DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol Precipitation for dGTP Terminators v1.0 and v3.0
Purification
Methods
• Ethanol/sodium acetate precipitation (below)
– dGTP terminators v3.0 (below)
– dGTP terminators v1.0 (below)
• Ethanol precipitation (below)
– Precipitating in 96-well reaction plates (page 106)
– Precipitating in microcentrifuge tubes (page 107)
Ethanol/Sodium
Acetate
Precipitation
dGTP Terminators v3.0 Only
Ethanol/sodium acetate precipitation produces consistent signal, and minimizes
unincorporated dyes. A final 70% ethanol wash is required.
Note: This method produces the cleanest signal, but it may cause loss of small
fragments.
For procedures, refer to the appropriate protocol:
• 96-well reaction plates or microcentrifuge tubes method – Refer to the dGTP
BigDye
®
Terminator v3.0 Ready Reaction Cycle Sequencing Kit Protocol
(PN 4390038).
• 384-well reaction plates method – Refer to the BigDye
®
Terminator v3.1 Cycle
Sequencing Kit Protocol (PN 4337035).
dGTP Terminators v1.0 Only
To perform ethanol/sodium acetate precipitation with dGTP terminators v1.0 kits,
refer to the dGTP BigDye
®
Terminator Ready Reaction Kit Protocol (PN 4307169).
Ethanol
Precipitation
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
11. (Optional) If you plan to store the plate before proceeding with
electrophoresis, seal the plate tightly with aluminum tape and store at
−20 °C protected from light.
12. Proceed to “Minimum Sample Volume” on page 122.
To precipitate 10-μL sequencing reactions in 384-well reaction plates: (continued)

106 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol precipitation improves the recovery of small fragments, but you may observe
residual terminator peaks resulting from unincorporated terminators precipitating
with the small fragments.
To precipitate 20-μL reactions in 96-well reaction plates:
1. Remove the 96-well MicroAmp
®
plate from the thermal cycler. Remove the
caps from each tube.
2. Add the following for each sample:
• 16 µL of deionized water
• 64 µL of non-denatured 95% ethanol
The final ethanol concentration should be 60 ± 3%.
3. Seal the tubes with strip caps or by applying a piece of self-adhesive film.
Press the film onto the tubes to prevent any leakage.
4. Invert the plate a few times to mix.
5. Leave the plate at room temperature for 15 minutes to precipitate the
extension products.
Note: Precipitation times <15 minutes result in the loss of very short
extension products. Precipitation times >24 hours increase the precipitation
of unincorporated dye terminators.
6. Place the plate in a tabletop centrifuge with a tube-tray adapter and spin it at
the maximum speed, which must be ≥1400 ✕ g but <3000 ✕ g:
• 1400 to 2000 ✕ g: 45 minutes
• 2000 to 3000 ✕ g: 30 minutes
Note: A MicroAmp tube in a MicroAmp plate can withstand 3000 ✕ g for
30 minutes.
IMPORTANT! Proceed to the next step immediately. If this is not possible,
then spin the tubes for an additional 2 minutes immediately before
performing the next step.
7. Without disturbing the precipitates, remove the adhesive tape and discard the
supernatant by inverting the plate onto a paper towel folded to the size of the
plate.
8. Place the inverted plate with the paper towel into the table-top centrifuge and
spin at 50 ✕ g for 1 minute.
9. Add 150 µL of 70% ethanol to each pellet.
10. Spin the plate for 10 minutes at maximum speed. See step 6 above.
11. Without disturbing the precipitates, remove the adhesive tape and discard the
supernatant by inverting the plate onto a paper towel folded to the size of the
plate.

107DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol Precipitation in Microcentrifuge Tubes
With ethanol precipitation, residual terminator peaks may be seen. However, the
recovery of small fragments is improved using this precipitation method because of
the higher g forces generated in a microcentrifuge, compared with a tabletop
centrifuge.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
12. Place the inverted plate with the paper towel into the table-top centrifuge and
spin at 50 ✕ g for 1 minute.
13. Remove the plate and discard the paper towel.
Note: Pellets may or may not be visible. Vacuum drying of the samples is
not necessary.
14. Proceed with “Minimum Sample Volume” on page 122.
To precipitate 20-μL reactions in microcentrifuge tubes:
1. Pipette the entire contents of each extension reaction into a 1.5-mL
microcentrifuge tube.
2. Add the following for each sample:
• 16 µL of deionized water
• 64 µL of non-denatured 95% ethanol
The final ethanol concentration should be 60 ± 3%.
3. Close the tubes and vortex them briefly.
4. Leave the tubes at room temperature for 15 minutes to precipitate the
extension products.
Note: Precipitation times <15 minutes result in the loss of very short
extension products. Precipitation times >24 hours increase the precipitation
of unincorporated dye terminators.
5. Place the tubes in a microcentrifuge and mark their orientations. Spin the
tubes for 20 minutes at maximum speed.
IMPORTANT! Proceed to the next step immediately. If this is not possible,
then spin the tubes for an additional 2 minutes immediately before
performing the next step.
To precipitate 20-μL reactions in 96-well reaction plates: (continued)

108 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
6. Carefully aspirate the supernatants with a separate pipette tip for each
sample and discard. Pellets may or may not be visible.
IMPORTANT! The supernatants contain unincorporated dye terminators.
You must remove the supernatants completely to remove unincorporated dye
terminators.
7. Add 250 µL of 70% ethanol to the tubes and vortex them briefly.
8. Place the tubes in the microcentrifuge in the same orientation as in step 5
and spin at maximum speed for 10 minutes.
9. Aspirate the supernatants carefully, as in step 6.
10. Dry the samples in a vacuum centrifuge for 10 to 15 minutes or to dryness.
Do not over-dry.
11. Proceed with “Minimum Sample Volume” on page 122.
To precipitate 20-μL reactions in microcentrifuge tubes: (continued)

109DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol Precipitation for dRhodamine Terminators
Purification
Methods
• Ethanol/sodium acetate precipitation (below)
• Shrimp alkaline phosphatase digestion (page 110)
• Simplified ethanol precipitation:
– Precipitating in microcentrifuge tubes (page 111)
– Precipitating in MicroAmp trays (page 112)
Ethanol/Sodium
Acetate
Precipitation
Because sequencing reactions using dRhodamine terminators produces less signal
than reactions using BigDye terminators, take care when performing ethanol
precipitation of sequencing reactions using dRhodamine terminators. With ethanol
precipitation, you may observe minimal traces of unincorporated terminators at the
beginning of the sequence data up to base 40. You may also observe loss in the
recovery of the smallest fragments.
CHEMICAL HAZARD. 3 M sodium acetate may cause eye,
skin, and respiratory tract irritation. Read the MSDS, and follow the handling
instructions. Wear appropriate protective eyewear, clothing, and gloves.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
To perform ethanol/sodium acetate precipitation (20-μL reactions):
1. For each sequencing reaction, prepare a 1.5-mL microcentrifuge tube
containing:
• 2.0 µL of 3 M sodium acetate (NaOAc), pH 4.6
• 50 µL of 95% ethanol (EtOH)
Note: The final ethanol concentration should be ~66%.
2. Precipitate the extension products:
a. Pipette the entire contents of each extension reaction into each tube of
sodium acetate/ethanol mixture. Mix each tube thoroughly.
b. Vortex the tubes and place them on ice for 10 minutes.
3. Pellet the precipitated extension products:
a. Centrifuge the tubes at maximum speed for 15 to 30 minutes.
b. Carefully aspirate the supernatant with a pipette, then discard the
supernatant.

110 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Shrimp Alkaline
Phosphatase
Digestion
(Optional)
The following shrimp alkaline phosphatase (SAP) digestion procedure can be used
for more efficient removal of unincorporated dye terminators, but is optional. If you
use this procedure for dye removal, then you must perform a simplified ethanol
precipitation (below) afterwards.
Simplified
Ethanol
Precipitation
These protocols require 70% ethanol (EtOH) containing 0.5 mM MgCl
2
. This
reagent can be prepared in situ or as a stock solution. Two protocols are given below:
• Precipitating in microcentrifuge tubes (below)
• Precipitating in MicroAmp trays (page 112)
IMPORTANT! Do not perform this protocol when purifying extension products with
any of the BigDye terminators, because magnesium interferes with the removal of
unincorporated BigDye terminators.
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
CHEMICAL HAZARD. Magnesium chloride may cause eye,
skin, and respiratory tract irritation, central nervous system depression, and kidney
damage. Read the MSDS, and follow the handling instructions. Wear appropriate
protective eyewear, clothing, and gloves.
4. Perform a 70% ethanol rinse:
a. Add 250 µL of 70% ethanol to the pellet.
b. Centrifuge the pellet at maximum speed for 5 minutes.
c. Carefully aspirate the supernatant with a pipette, then discard the
supernatant.
5. Dry the pellet in a vacuum centrifuge for 10 to 15 minutes, or until dry. Do
not over-dry the pellet.
6. Proceed with “Minimum Sample Volume” on page 122.
To perform ethanol/sodium acetate precipitation (20-μL reactions): (continued)
To perform shrimp alkaline phosphatase (SAP) digestion:
1. At the end of thermal cycling, add 2 µL of SAP (1 U/µL) and 18 µL of 1 ✕
SAP buffer to each tube. Seal each tube and incubate them at 37 °C for
30 minutes.
2. Proceed with simplified ethanol precipitation:
• To precipitate in microcentrifuge tubes, see the procedure below.
• To precipitate in MicroAmp trays, go to page 112.

111DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
To precipitate in microcentrifuge tubes:
1. Pipette the entire contents of each extension reaction into a 1.5-mL
microcentrifuge tube.
2. Select one method for adding the 70% ethanol (EtOH)/0.5 mM MgCl
2
solution.
Add the reagents directly:
• For reactions after SAP digestion – Add 40 µL of 2 mM MgCl
2
and
then 110 µL of 95% ethanol.
• For reactions that do not use SAP digestion – Add 20 µL of 2 mM
MgCl
2
and then 55 µL of 95% ethanol.
Create an EtOH/MgCl
2
stock solution and add it:
a. Combine the following in a 1.5-mL microcentrifuge tube:
–1mL 70% EtOH
–1µL 0.5M MgCl
2
b. Vortex briefly.
c. Add the stock solution:
– For reactions after SAP digestion – Add 150 µL of the
EtOH/MgCl
2
stock solution to each tube.
– For reactions that do not use SAP digestion – Add 74 µL of the
EtOH/MgCl
2
stock solution to each tube.
3. Close the tubes and vortex them briefly.
4. Leave the tubes at room temperature for 10 to 15 minutes to precipitate the
extension products.
Note: Precipitation times less than 5 minutes result in the loss of very short
extension products. Precipitation times greater than 24 hours increase the
precipitation of unincorporated dye terminators.
5. Place the tubes in a microcentrifuge and mark their orientations. Centrifuge
the tubes at maximum speed for 10 to 20 minutes.
IMPORTANT! Proceed to the next step immediately.
6. Carefully aspirate and discard the supernatants with a separate pipette for
each sample. Pellets may or may not be visible.
IMPORTANT! The supernatants must be removed completely because
unincorporated dye terminators are dissolved in them. The more residual
supernatant left in the tubes, the more unincorporated dye terminators
remain in the samples.
7. Visually inspect the sample tubes for residual supernatants. If there are any
residual supernatants:
a. Place the tubes in the microcentrifuge in the same orientation as in
step 5 and spin for 5 to 10 seconds.
b. Aspirate the supernatants carefully as in step 6.

112 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
8. Dry the samples in a vacuum centrifuge for 10 to 15 minutes or to dryness.
(Or, place the tubes with the lids open in a heat block or thermal cycler at
90 °C for 1 minute.)
To precipitate in MicroAmp
®
trays:
1. Remove the MicroAmp Tray from the thermal cycler. Remove the caps from
each tube.
2. Select one method for adding the 70% ethanol (EtOH)/0.5 mM MgCl
2
solution.
Add the reagents directly:
• For reactions after SAP digestion – Add 40 µL of 2 mM MgCl
2
and
then 110 µL of 95% ethanol.
• For reactions that do not use SAP digestion – Add 20 µL of 2 mM
MgCl
2
and then 55 µL of 95% ethanol.
Create an EtOH/MgCl
2
stock solution and add it:
a. Combine the following in a 1.5-mL microcentrifuge tube:
–1mL 70% EtOH
–1µL 0.5M MgCl
2
b. Vortex briefly.
c. Add the stock solution:
– For reactions after SAP digestion – Add 150 µL of the
EtOH/MgCl
2
stock solution to each tube.
– For reactions that do not use SAP digestion – Add 74 µL of the
EtOH/MgCl
2
stock solution to each tube.
3. Seal the tubes by applying a piece of 3M Scotch Tape 425 adhesive-backed
aluminum foil tape. Press the foil onto the tubes to prevent any leakage.
4. Invert the tray a few times to mix.
5. Leave the tray at room temperature for 10 to 15 minutes to precipitate the
extension products.
Note: Precipitation times less than 5 minutes result in the loss of very short
extension products. Precipitation times greater than 24 hours increase the
precipitation of unincorporated dye terminators.
6. Place the tray in a table-top centrifuge with tube-tray adaptor for 20 to
30 minutes at the maximum speed (at least 1400 ✕ g but less than
3000 ✕ g).
Note: A MicroAmp tube in a MicroAmp Tray can withstand 3000 ✕ g for
30 minutes.
IMPORTANT! Proceed to the next step immediately.
To precipitate in microcentrifuge tubes: (continued)

113DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
7. Without disturbing the precipitates, remove the adhesive tape and discard the
supernatant by inverting the tray onto a paper towel folded to the size of the
tray.
8. Place the inverted tray with the towel into the table-top centrifuge and spin at
500 to 1000 ✕ g for 1 minute.
9. Remove the tray and discard the paper towel.
Note: Pellets may or may not be visible. Vacuum drying of the samples is
not necessary.
10. Proceed with “Minimum Sample Volume” on page 122.
To precipitate in MicroAmp
®
trays: (continued)

114 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Ethanol Precipitation for BigDye Primers
Required
Materials
Reagents and equipment required:
• 1.5-mL microcentrifuge tubes
• Benchtop microcentrifuge, capable of reaching at least 14000 ✕ g
• Vacuum centrifuge
• 95% Ethanol (ACS reagent grade), non-denatured
Note: This method does not use salt.
Ethanol
Precipitation
CHEMICAL HAZARD. Ethanol is a flammable liquid and
vapor. Exposure causes eye, skin, and respiratory tract irritation and may cause
central nervous system depression and liver damage. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
1. Select one method for adding the 95% ethanol and combining the reactions
into one tube.
Transfer the extension reactions to a tube containing 95% ethanol:
a. Add 53 µL of 95% ethanol (100 µL if sequencing BAC DNA or other
high-sensitivity reactions) to a clean microcentrifuge tube.
Note: Sodium acetate is not necessary for precipitation.
b. Pipette the extension reactions from the bottom of each of the four
tubes into the ethanol mixture. Mix thoroughly.
or
Add 95% ethanol to one reaction, then transfer the mixture to another
reaction:
a. Add 53 µL of 95% ethanol (100 µL if sequencing BAC DNA or other
high-sensitivity reactions) to the “A” reaction tube.
b. Transfer the contents of the “A” reaction tube into the “C” reaction
tube.
c. Pipette the mixture from the “C” reaction tube into the “G” reaction
tube and so on until the contents of all four reaction tubes are combined
in one 1.5-mL microcentrifuge tube.
2. Place the tube on wet ice or leave it at room temperature for 10 to 15 minutes
to precipitate the extension products.
3. Centrifuge the tube at maximum speed for 10 to 20 minutes. Carefully
aspirate the supernatant and discard. At this point, a pellet may or may not
be visible.
4. (Optional) Rinse the pellet with 250 µL of 70% ethanol and centrifuge for
5 minutes. Carefully aspirate the supernatant and discard. This rinse step
may remove some of the salts from the pellet but is often not necessary.
Note: If you use sodium acetate, rinse the pellet to reduce the carryover of
salt.

116 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Purification with Spin Columns and Spin Plates
Overview
1. Perform SDS/heat treatment of extension products (required for BigDye
terminators v3.1 and dGTP BigDye terminators v3.0) (below).
2. Prepare the spin column (page 117) or the spin plate (see manufacturer’s
instructions).
3. Perform purification with the spin column (page 118) or with the spin plate
(page 118).
Performing
SDS/Heat
Treatment
Perform this step to prepare extension products for both spin column and 96-well
spin plate purification. This SDS/heat treatment effectively eliminates
unincorporated dye terminators from cycle sequencing reactions.
IMPORTANT! This step is required for BigDye terminators v3.1 and dGTP BigDye
terminators v3.0 but is not required for BigDye terminators v1.1 or dGTP BigDye
terminators v1.0.
CHEMICAL HAZARD. Sodium dodecyl sulfate (SDS).
Exposure causes eye, skin, and respiratory tract irritation. Exposure may cause
severe allergic respiratory and skin reaction. Read the MSDS, and follow the
handling instructions. Wear appropriate protective eyewear, clothing, and gloves.
Recommended
Spin Columns
We recommend Centri-Sep
™
spin columns (Within the US: Princeton Separations;
Outside the US only: Applied Biosystems, PN 401763 for 32 columns and
PN 401762 for 100 columns).
To prepare extension products (20-μL reactions):
1. Prepare 2.2% SDS (sodium dodecyl sulfate) in deionized water. This SDS
solution is stable at room temperature.
2. Add an appropriate amount of the 2.2% SDS solution to each sample to
bring the final SDS concentration to 0.2%.
For example: Add 2 μL of 2.2% SDS to each 20-μL completed cycle
sequencing reaction.
3. Seal the tubes and mix thoroughly, then centrifuge the tubes at 1000 rpm for
1 minute to spin down the contents.
4. Heat the tubes to 98 °C for 5 min, then allow the tubes to cool to ambient
temperature before proceeding to the next step.
Note: A convenient way to perform this heating/cooling cycle is to place the
tubes in a thermal cycler and set it as follows:
•98°C for 5 min
• 25 °C for 10 min
5. Centrifuge the tubes briefly to spin down the contents.
6. Continue with spin-column or 96-well spin plate purification.

117DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Optimizing Spin
Column
Purification
Directions below apply only when using Centri-Sep individual spin columns.
IMPORTANT! When using the BigDye terminators v3.1, hydrate the column for
2 hours (see step 3 below).
Tips for optimizing spin column purification when using individual columns:
• Do not process more columns than you can handle conveniently at one time.
• Load the sample in the center of the column bed slowly. Make sure that the
sample does not touch the sides of the column and that the pipette tip does not
touch the gel surface.
Note: If you do not load the samples properly, you may not remove sufficient
unincorporated dye terminators and you may observe peaks from them in the
electropherogram. Failure to remove all of the SDS can result in damaged capillaries.
• Spin the column at 325 to 730 ✕ g for best results. Use the following formula to
calculate the best speed for your centrifuge:
g = 1.12 ✕ r ✕ (rpm/1000)
2
where:
g = relative centrifugal force
r = radius of the rotor in mm
rpm = revolutions per minute
• Do not spin for more than 2 minutes.
• Perform the entire procedure without interruption to ensure optimal results. Do
not allow the column to dry out.
Preparing the
Spin Column
To prepare the spin column:
1. Prepare the extension products according to “Performing SDS/Heat
Treatment” on page 116.
2. Tap the column gently to cause the gel material to settle to the bottom of the
column.
3. Hydrate the column:
a. Remove the upper end cap and add 0.8 mL of deionized water.
b. Replace the upper end cap and vortex or invert the column a few times
to mix the water and gel material.
c. Allow the gel to hydrate at room temperature for at least 2 hours.
Note: You can store hydrated columns for a few days at 2 to 6 °C. Longer
storage in water is not recommended. Allow columns stored at 2 to 6 °C to
warm to room temperature before use.
4. Remove any air bubbles by inverting or tapping the column and allowing the
gel to settle.
5. Remove the upper end cap first, then remove the bottom cap. Allow the
column to drain completely by gravity.
Note: If flow does not begin immediately, apply gentle pressure to the
column with a pipette bulb.

118 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Purifying with the
Spin Column
Performing Spin
Plate Purification
For large-scale procedures, you can use spin plates, such as DTR kits from Edge
Biosystems, Montage Filter Plates from Millipore, or Centri•Sep Multi-Well Filter
Plates from Princeton Separations.
Note: You may use other spin plate systems to remove unincorporated dye
terminators. However, because of the large number of variables associated with using
spin plate systems, optimize the performance of your system in your own laboratory.
Purifying with a
Spin Plate
6. Insert the column into the wash tube provided.
7. Centrifuge the column at 730 ✕ g for 2 minutes to remove the interstitial
fluid.
8. Remove the column from the wash tube and insert it into a sample collection
tube (for example, a 1.5-mL microcentrifuge tube).
To prepare the spin column: (continued)
To perform purification with the spin column:
1. Remove the extension reaction/SDS mixture from its tube and load it
carefully onto the center of the gel material.
2. Centrifuge the column at 730 ✕ g for 2 minutes.
Note: If you use a centrifuge with a fixed-angle rotor, the surface of the gel
will be at an angle in the column after the first spin. After the first spin,
return the column to its original orientation.
3. Discard the column. The sample is in the sample collection tube.
4. Dry the sample in a vacuum centrifuge without heat or at low heat for 10 to
15 minutes or until dry. Do not over-dry.
To perform purification with the spin plate:
1. Prepare the extension products according to “Performing SDS/Heat
Treatment” on page 116.
2. Prepare the spin plate per the manufacturer’s instructions.
3. Perform the purification per the manufacturer’s instructions.

119DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Alternative Purification Procedures
Other manufacturers provide products for removing unincorporated dye terminator
nucleotides, primers, excess salts, and other contaminants from sequencing reactions.
Table 13 lists some commercially available alternatives. Applied Biosystems has no
specific recommendations on the use of these products. Refer to the manufacturer’s
documentation for instructions.
Table 13 Alternative purification methods for extension products
Product Source
Size Exclusion Products
Performa DTR Ultra 96-Well Plates Edge Biosystems
Sephadex G-50 or Sephadex G-75 columns Major laboratory suppliers
Montage SEQ
96
and SEQ
384
Filter Plates Millipore
MultiScreen SEQ
384
Filter Plates Millipore
Centri•Sep Columns Princeton Separations
Centri•Sep 96 Multi-Well Filter Plates Princeton Separations
DyeEx 2.0 Spin Kit or DyeEx 96 Kit QIAGEN
SigmaSpin 96-Well Post-Reaction Clean-Up Plates Sigma-Aldrich
SigmaSpin Post-Reaction Clean-Up Columns Sigma-Aldrich
Magnetic Bead Adsorption/Elution
CleanSEQ Agencourt
MagDTR Magnetic Resin Edge Biosystems
Wizard MagneSil Sequencing Reaction Clean-Up System Promega

120 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Sample Preparation for Electrophoresis
This section has instructions for preparing purified samples for electrophoresis.
Choose the instructions based on how your sample was purified:
• BigDye XTerminator Purification kit, see below
• Other purification methods, see page 121
Samples Purified with the BigDye XTerminator Purification Kit
Storage of
Purified Samples
Sequencing reactions cleaned with BigDye XTerminator can be stored under the
following conditions:
• Room temperature – Plates sealed with heat seal film, adhesive film, or septa
can be stored for up to 48 hours at room temperature (20 to 25 °C).
• Refrigerated storage – Plates sealed with heat seal film or adhesive film can be
stored for up to 10 days at 4 °C (recommended).
• Frozen storage – Plates sealed with heat seal film or adhesive film can be
stored for up to 1 month at –20 °C.
Minimum Sample
Volume
Because there is a high concentration of dye-labeled sequencing fragments, one
sample can be injected several times because very little sample volume is used for
each injection. Make sure that you obtain the minimum sample volume so that the
ends of the capillaries remain submerged in liquid during injection. The minimum
sample volume is 10 µL for 96-well plates and 5 µL for 384-well plates.
Note: Because injection is electrokinetic, the resuspension volume does not affect
the signal as it does for slab gel electrophoresis.
Resuspension
Solutions
Sequencing reaction products purified with BigDye XTerminator do not require any
additional resuspension solutions.
Note: Do not heat samples or add Hi-Di
™
Formamide when using BigDye
XTerminator to purify samples.

121DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Sample
Preparation
Procedure
Samples Purified with Other Purification Methods
Storing
Sequencing
Reactions
You can store dried, cleaned up, and sealed sequencing reactions at –20 °C for many
days.
IMPORTANT! Protect fluorescently labeled DNA from light, heat, acidic conditions,
and oxygen to prevent dye degradation.
To prepare the plate after BigDye XTerminator purification:
1. Place the reaction plate in the genetic analyzer:
2. Select the appropriate BigDye XTerminator run module for your instrument
and plate type (see “Selecting a Run Module” on page 137).
Note: Use standard run modules if you transferred the supernatant to a clean
plate after centrifuging.
Plate
Type
Instrument
Seal Used for
Vortexing
Instructions
96-well 3730/3730xl Heat seal Place the plate directly in the
instrument.
MicroAmp
®
Clear
Adhesive Film
Remove the seal, replace it with
a septa mat, then place the
plate in the instrument.
3100/3100-
Avant
‡
,
3130/3130xl
Either Remove the seal, replace it with
a septa mat, then place the
plate in the instrument.
310 Genetic
Analyzer
Either Transfer 10 µL of supernatant to
a clean plate, cover the plate
with a septa mat, then place the
plate in the instrument.
384-
well
3730/3730xl Heat seal Place the plate directly in the
instrument.
MicroAmp
Clear
Adhesive Film
• Remove the clear adhesive
film, replace it with a heat
seal, then place the plate in
the instrument.
or
• Transfer 10 µL of supernatant
to a clean plate, cover with a
septa mat, then place the
plate in the instrument.
3100/3100-
Avant
‡
,
3130/3130xl, 310
Genetic Analyzer
Either Transfer 10 µL of supernatant to
a clean plate, cover with a septa
mat, then place in the
instrument.
‡ The instrument must be using Data Collection Software v2.0 or later. If not, transfer
10 µL of supernatant to a clean plate, cover the plate with a septa mat, then place
the plate in the instrument.

122 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products
Minimum Sample
Volume
Minimum sample volume is the volume needed so that the ends of the capillaries
remain submerged in liquid during injection. The minimum sample volume is 10 μL
for 96-well plates and 5 μL for 384-well plates. Because the sample is transferred to
the capillary by electrokinetic injection, the sample volume does not change and you
can inject each sample multiple times.
Resuspension
Solutions
Applied Biosystems recommends using Hi-Di Formamide to resuspend your purified
sequencing products. For purification methods that result in extension products in
water, Applied Biosystems recommends drying the sample in a speed vacuum and
then resuspending the dried sample in Hi-Di Formamide.
If you choose to resuspend your samples in formamide not purchased from Applied
Biosystems, make sure that you use only high-quality formamide. Also, Applied
Biosystems recommends that you eliminate the denaturation step. Heating samples
that are resuspended in formamide may result in dye degradation and shoulders on all
peaks (page 234).
Resuspension in water is not recommended because oxidative effects on terminator
dyes lead to earlier dye breakdown of sequencing extension products, affecting
basecalling.
Sample
Preparation
Procedure
To prepare samples for capillary electrophoresis on a 310 instrument, see the
BigDye
®
Terminator v3.1 Sequencing Standard Product Insert (PN 4336878).
CHEMICAL HAZARD. Formamide. Exposure causes eye,
skin, and respiratory tract irritation. It is a possible developmental and birth defect
hazard. Read the MSDS, and follow the handling instructions. Wear appropriate
protective eyewear, clothing, and gloves.
To prepare samples for capillary electrophoresis on 31XX and 37XX instruments:
1. Add 10 μL Hi-Di
™
Formamide to each sample pellet:
IMPORTANT! To prevent dye degradation:
• Use fresh Hi-Di Formamide. Old Hi-Di Formamide or low quality
formamide can have formic acid that can contribute to the degradation
of fluorescent dyes.
• Run samples on the instrument as soon as possible after resuspending
them.
2. Seal the wells with aluminum sealing tape.
3. Vortex thoroughly, then centrifuge briefly.
4. Peel off the aluminum sealing tape and replace the tape with either septa or a
heat seal.
Note: You must use tube septa or a heat seal to prevent exposure to air and
evaporation of samples, especially if you place the samples in the
autosampler more than 6 hours before starting electrophoresis.
Note: Heat seals are available for 3730/3730xl instruments only.

123DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products

124 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 5 Purification of Extension Products

Part Number 4305080 Rev. C 05/2009

127DNA Sequencing by Capillary Electrophoresis Chemistry Guide
6
Capillary Electrophoresis 6
This chapter covers:
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .128
Calibrating the Instrument. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .131
Creating the Plate Record in Data Collection Software . . . . . . . . . . . . . . . . . . . . .136
Defining the Results Group. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .136
Defining the Instrument Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .137
Defining the Analysis Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .143
Run Parameters for Instrument and Analysis Protocols . . . . . . . . . . . . . . . . . . . . .151

128 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Overview
This chapter provides an overview of capillary electrophoresis on Applied
Biosystems instruments for automated DNA sequencing. For more information, refer
to your instrument user guide.
Workflow
DNA Template Preparation (Chapter 3)
Cycle Sequencing (Chapter 4)
Extension Product Purification (Chapter 5)
Capillary Electrophoresis
1. Prepare the instrument
2. Calibrate the instrument
3. Set up the plate record in Data
Collection Software:
– Results group
– Instrument protocol
– Analysis protocol
4. Load samples.
5. Perform the run.
Capillary Electrophoresis Output:
Applied Biosystems instruments:
Data Analysis (Chapter 7)
*.ab1 file
3730/3730xl analyzer
3100/3100-Avant analyzer
ABI PRISM
1
2
3
4
5
B-D
310 analyzer
3130/3130xl analyzer

129DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Electrophoresis Workflow
Follow the general flow below to perform a run on Applied Biosystems genetic
analyzers. For more information and procedures, refer to your instrument user guide.
Instrument Consumables
Sequencing
Standards
The cycle sequencing standards provide an additional control for troubleshooting
electrophoresis runs because they can help you distinguish between chemistry
problems and instrument problems. These standards contain lyophilized prelabeled
sequencing reactions that require only resuspension before use. Standards available:
•BigDye
®
Terminator v3.1 Sequencing Standard (3730, 3730xl) (PN 4336743)
•BigDye
®
Terminator v3.1 Sequencing Standard (310, 3100-Avant, 3100, 3130,
3130xl) (PN 4336935)
•BigDye
®
Terminator v1.1 Sequencing Standard (3730, 3730xl) (PN 4336799)
1. Prepare the instrument:
a. Start the computer, the instrument, and the Data Collection Software.
b. Select the capillary length, number of capillaries, and polymer type
(page 130).
c. Check the polymer delivery system for sufficient polymer and
absence of bubbles.
d. Prepare the buffer and water/waste reservoirs.
2. Perform spatial calibration, if necessary (page 131).
3. Perform spectral calibration, if necessary (page 131).
4. Load the prepared samples:
a. (Multicapillary instruments only) Load the prepared samples onto 96-
well or 384-well plates.
b. Place the tubes or the plate assembly into the instrument.
5. Set up the run using Data Collection Software:
a. Create a plate record (page 136).
b. Create a results group, with file name and folder preferences
(page 136).
c. Create an instrument protocol, including run module and dye set
configurations (page 137).
d. Create an analysis protocol (page 143).
e. Determine the run parameters (page 151).
f. (Multicapillary instruments only) Link the plate.
6. Start the run.

130 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
•BigDye
®
Terminator v1.1 Sequencing Standard (310, 3100-Avant, 3100, 3130,
3130xl) (PN 4336791)
Note: For preparation of these standards, refer to the product inserts.
POP
™
Polymer
Applied Biosystems genetic analyzers use POP
™
Performance Optimized Polymer to
separate DNA fragments. Use only the polymer recommended for your instrument.
IMPORTANT! Do not leave polymer on the instrument more than 7 days.
Applied Biosystems recommends that you minimize actions that could introduce
bubbles or particles into the polymer. Introduction of dust into the polymer can cause
spikes in the data. To minimize bubbles and particles:
• Make sure that the polymer cap is closed to minimize exposure of the polymer
to air during storage.
• Clean the polymer delivery system with deionized water.
• Discard capillaries that are exposed to dust or are dried out.
• Change the buffer and water and discard the waste daily or before each set of
runs.
Capillaries
In all Applied Biosystems capillary instruments, the capillary has an opaque,
polyamide external coating except in the detection window area. During
electrophoresis, the laser and detector read samples through the window in the
coating. Capillaries are very fragile in the uncoated window area.
If treated properly, capillaries are guaranteed for 300 runs for 3730/3730xl
instruments, for 150 runs for 3130 and 3100-Avant instruments, and for 100 runs for
3130xl, 3100, and 310 instruments. You may be able to get more injections from a
capillary, depending on your template preparation methods and run conditions.
• Store the capillary ends in buffer or deionized water when not in use to prevent
the capillary ends from drying out.
• Store unused capillaries in a dust-free environment.
• Do not touch capillary windows. If you accidentally touch a window, clean it
according to the procedure in your instrument user guide.
Possible signs of capillary failure:
• Gradual loss of resolution (see page 251)
• High baseline
• Noisy data or peaks under peaks throughout the sequence (see page 237)
• Shoulders on peaks or irregular peaks (see pages 231, 233, and 234)
Genetic Analyzer
Running Buffer
Use only Applied Biosystems genetic analyzer running buffer for electrophoresis.
Other running buffers may not perform well and using them may void warranty or
service contracts. Applied Biosystems genetic analyzer running buffers are supplied
at 10✕ concentrations. Dilute to 1✕ with distilled water before use:
• 3730/3730xl instruments – Applied Biosystems 3730/3730xl 10✕ Running
Buffer with EDTA (PN 4335613)
• 3130/3130xl, 3100/3100-Avant instruments, and 310 instruments –
10✕ Genetic Analyzer Buffer with EDTA (PN 402824)

131DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Calibrating the Instrument
Multiple capillary instruments require calibration before performing a sample run to
maximize data quality and accuracy.
Spatial Calibration
The Data Collection Software uses images collected during the spatial calibration to
map each signal detected by the CCD camera to a position in the capillary array.
Spatial calibration is required for all multi-capillary instruments.
When to Perform
You are required to perform a spatial calibration when you:
• Install or replace a capillary array
• Temporarily remove the capillary array from the detection block
• Move the instrument
• Open the detection block
Spectral Calibration
Performing a spectral calibration is similar to performing a sample run. When you
perform a spectral calibration, you run spectral calibration standards as samples and
use a spectral calibration module as a run module. The results from a spectral
calibration run are used to create a matrix of spectral values that define the spectral
overlap between the different dyes.
Note: If you are using the 310 instrument, create a matrix file manually after
performing the matrix run.
The values in the matrix are unique for each instrument, for each dye set, and for
each specific set of run conditions. Data Collection Software applies the values in the
matrix to the sample data to perform multicomponent analysis: the reduction of raw
data from the instrument to the 4-dye data stored in sequencing files.
When to Perform
You must perform a spectral calibration run:
• When you use a new dye set on the instrument
• When you change the capillary array or polymer
• For each combination of capillary array length and each sequencing dye set that
you use
• After the laser or CCD camera has been realigned/replaced by a service
engineer
• If you begin to observe a decrease in the quality of raw or analyzed data (for
example, pull-up and/or pull-down peaks with a distinct pattern)

132 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Spectral Calibration Standard Types
There are two types of spectral calibration standards:
• Matrix standards for sequencing – A tube that contains four different
fragments, each labeled with a different single dye.
Note: Matrix standards are not designed for use on 3730 instruments. Use
sequencing standards for spectral calibration of 3730 instruments.
• Sequencing standards – A tube of a standard chemistry reaction that contains
multiple fragments labeled with the four dyes.
Parameter Files
for Spectral
Calibration
The Data Collection Software uses a parameter file to process the data to create a
matrix. Each parameter file contains an algorithm to process the source data and
information to evaluate the final matrix.
Table 14 describes when to use each type of parameter file.
During the Calibration
Matrix standards are run in all capillaries. The Data Collection Software collects the
data and stores it into separate temporary files. The Data Collection Software then
analyzes the data, then generates and stores a mathematical description of the
spectral overlap for each capillary.
Dye Set and
Spectral
Calibration
Standards
Use Table 15 to determine the correct dye set and spectral calibration standard for the
cycle sequencing kit you use. Note that the standards are instrument-specific.
A dye set is a place holder for spectral information for a given set of four dyes.
Note: Because different calibration algorithms are used for Big Dye Terminator v3.1
and Big Dye Terminator v1.1, the matrix standards are not interchangeable
.
Table 14 Spectral calibration criteria
Dye Parameter File
For Each
Dye…
Q Value
Condition
Bounds
Matrix standard set DS-01 MtxStd{Sequencing SetE}.par Single peak 0.92 3.0 to 5.0
BigDye Terminator standard or
sequencing reaction
SeqStd{Sequencing-SetE}.par Multiple
peaks
0.95 3.0 to 5.0
Matrix standard set for BigDye
v1.1
MtxStd{Sequencing-SetE}.par Single peak 0.95 3.0 to 5.0
BigDye Terminator v3.1 standard
or sequencing reaction
SeqStd{Sequencing-SetZ}.par Multiple
peaks
0.95 3.0 to 5.0
Matrix standard-unknown dye set MtxStd{AnyDyeSet}.par Single peak 0.95 None specified
A sequencing reaction that uses a
4-color dye set other than dye set
E/Z
SeqStd{AnyDyeSet}.par Multiple
peaks
0.95 None specified

133DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Table 15 Dye sets and spectral calibration standards
Cycle Sequencing Kit Dye Set Spectral Calibration Standard
‡
•BigDye
®
Terminator v3.1 Cycle Sequencing Kits
•dGTP BigDye
®
Terminator v3.0 Cycle
Sequencing Kits
Z Matrix standards:
•BigDye
®
Terminator v3.1 Matrix Standard (3100-
Avant, 3100, 3130, 3130xl) (PN 4336974)
•BigDye
®
Terminator v3.1 Matrix Standards (310)
(PN 4336948)
Sequencing standards:
•BigDye
®
Terminator v3.1 Sequencing Standard
(3730, 3730xl) (PN 4336943)
•BigDye
®
Terminator v3.1 Sequencing Standard
(3130, 3130xl, 3100-Avant, 3100, 310)
(PN 4336935)
•BigDye
®
Terminator v1.1 Cycle Sequencing Kits
•BigDye
®
Primer Cycle Sequencing Kits
•dGTP BigDye
®
Terminator Cycle Sequencing
Kits
E Matrix standards:
•BigDye
®
Terminator v1.1 Matrix Standard (3100-
Avant, 3100) (PN 4336824)
•BigDye
®
Terminator v1.1 Matrix Standards (310)
(PN 4336805)
Sequencing standards:
•BigDye
®
Terminator v1.1 Sequencing Standard
(3730, 3730xl) (PN 4336799)
•BigDye
®
Terminator v1.1 Sequencing Standard
(3130, 3130xl, 3100-Avant, 3100, 310)
(PN 4336791)
dRhodamine Terminator Cycle Sequencing Kits E dRhodamine Matrix Standards Kit (310)
(PN 403046, 4 tubes; PN 403047, 10 reactions)
Long Read dRhodamine Terminator Cycle
Sequencing Standard (PN 4303120)
‡ Matrix standards are not designed for use on 3730 instruments. Use sequencing standards for spectral calibration of
3730 instruments.

134 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Evaluating the
Calibration
Results
Evaluate the Data
Use the following criteria to evaluate the data:
• Q Value – A value which measures the consistency between the final matrix and
the data from which it was computed. When the Q value is 1.0, the fit is perfect.
The matrix is ideal and there were no detected pull-up or pull-down peaks.
• Condition Number – The condition number is a value that represents the
amount of overlap between the dye peaks in the emission spectra of the dyes in a
dye set.
• Spectral profile – The spectral profile shows the emission spectra of the four
dyes.
• Raw data – The emission image exhibits four distinct fluorescence emission
maxima, one for each dye, if matrix standards are used (Figure 31). If
sequencing standards are used, there are multiple peaks (Figure 32).
Figure 31 Example output from a spectral calibration using matrix standards
Q Value
Condition Number
Spectral profile
Raw data from the
selected capillary

135DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Figure 32 Example output from a spectral calibration using sequence standards
Corrective Actions and Troubleshooting
If the spectral calibration failed, or if you did not like the appearance of a calibration
that passed, try one or more of the following:
• Check instrument status for any run errors.
• Verify the correct parameter file and run module were selected (see Table 14 on
page 132). Correct and repeat run.
• Check the freshness and preparation of reagents.
• Check for possible contamination of matrix standards.
• Make sure that there are no bubbles in the sample wells.
• Verify that all peaks were detected.
A slow running system can cut off the blue peak partially or entirely. Add time
to the run or change reagents (if they are suspect) and repeat the run.
• Use the Override Spectral Calibration command to replace a matrix with a
neighboring one.
Note: There may be instances when the spectral calibration passes in the software,
yet the quality of the calibration is not acceptable. In these instances, you can
override the calibration.
For troubleshooting, the spectral calibration log file is located in: E:\
AppliedBiosystems\UDC\DataCollection\data\instrument_model\
SpectralCalTmpFiles\Z, where instrument_model is the model of your instrument.
If you are creating a matrix on the 310 instrument, please refer to the ABI P
RISM
®
310 Genetic Analyzer User Guide (PN 4317588).
Q Value
Condition Number
Spectral profile
Raw data from the
selected capillary

136 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Creating the Plate Record in Data Collection Software
The Plate Record
Create a plate record for each plate to be run. A plate records consists of the
following information about the plate and all samples on the plate.
For detailed information about creating the plate record, see the appropriate user
guide for your instrument.
• Plate information:
– ID or barcode number (for 3730/3730xl instruments only)
– Plate name
– Plate type
– Application type
– Owner name
– Operator name
• For each sample:
– Sample name
– Comment (optional)
– Results group – Contains settings for file names and locations, the
sequencing analysis software in use, and the autoanalysis setting (see
page 136).
– Analysis protocol – Contains settings necessary for analysis and post-
processing of the sequence (see page 143).
– Instrument protocol – Contains settings for running the instrument (see
page 151).
Note: For 310 instruments, the injection list contains information about the samples,
not the plate record. Use the 310 Data Collections preferences to specify file names.
Defining the Results Group
Results Group
Contents
The results group defines:
• Results group name
• Analysis type
• Location for the *.ab1 sample files and any additional files generated by the
analysis
• Naming conventions to be used for sample files and the run folder
• AutoAnalysis setting
Only the Autoanalysis setting is described here.
Autoanalysis
Setting
In the Data Collection Software Results Group settings, choose whether to analyze
your sequencing files automatically, immediately after the electrophoresis run, or
manually.

137DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
• Autoanalysis – With autoanalysis, the software applies analysis protocols to
sequencing files immediately after Data Collection Software collects the data
from the instrument.
Note: Because autoanalysis is performed on the same computer that collects the
data, you must register the analysis software with the Data Collection Software.
• Manual analysis – With manual analysis, you obtain the sequencing files from
the computer connected to the instrument, then move or copy the files to another
computer that has Sequencing Analysis, Variant Reporter, or SeqScape
Software installed. To perform analysis, you apply the analysis protocols to the
sequencing files.
Defining the Instrument Protocol
Instrument
Protocol
Contents
The instrument protocol contains settings for running the instrument:
• Protocol name and type
• Run module
•Dye set
• Polymer
• Capillary array length
•Chemistry
Only the run module is discussed in detail in this section.
Selecting a Run Module
Run Module
Contents
The run module contains settings required for electrophoresis, including:
• Oven temperature
• Prerun voltage and time
• Injection voltage and time
• Run voltage settings
• Current settings
•Run time
Available Run
Modules
Use the tables in this section to select a run module based on the desired read length
and the run time for your sequencing chemistry. Choosing the incorrect run module
can result in data starting earlier or later than expected (see page 213).
• Table 16, “3730/3730xl instrument run modules,” on page 138
• Table 17, “3130/3130xl instrument run modules,” on page 138
• Table 18, “3100 instrument run modules,” on page 139
• Table 19, “3100-Avant instrument run modules,” on page 139

138 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
• Table 20, “310 instrument run modules,” on page 140
• Table 21, “BigDye® XTerminator™ Purification Kit run modules,” on
page 140
Note: In Tables 16 through 20, read lengths are based on BigDye Terminator v3.1
sequencing standards. These read lengths are minimum specifications for BigDye
Terminator v3.1 sequencing standards. Average base spacing values for the KB
basecaller and the ABI basecaller range from 10 to 24.
Table 16 3730/3730xl instrument run modules
Run Module
Array Length
(cm)
Run Time
(minutes)
Throughput (samples/day) Read Length (bases)
3730
Analyzer
3730xl
Analyzer
ABI
Basecaller
‡
KB
Basecaller
§
Ta rg e tS eq
™
Resequencing System
36 20 3456
#
6912
#
NA 400
Rapid sequencing 36 35 1920 3840 500 550
Standard sequencing 36 60 1152 2304 650 700
Fast sequencing 50 60 1152 2304 NA 700
Long sequencing 50 120 576 1152 800 850
Extra long sequencing 50 180 384 768 NA 900
‡ Read length using the ABI basecaller with 98.5% basecalling accuracy.
§ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).
# Throughput numbers for 400bp reads (Long read standards—HSP69 template). The run module can be customized to
run 200-400 bases.
Table 17 3130/3130xl instrument run modules
Run Module
Array Length
(cm)
Polymer
Run Time
(minutes)
Throughput (samples/day)
Read
Length
‡
(bases)
3130
Analyzer
3130xl
Analyzer
Ultra rapid sequencing 36 POP-4 40 144 576 400
Rapid sequencing 36 POP-6 60 96 384 500
Ultra rapid sequencing 36 POP-7 35 164 656 500
Rapid sequencing 36 POP-7 60 96 384 600
Standard sequencing 50 POP-4 100 56 224 600
Standard sequencing 50 POP-6 150 36 144 600
Fast sequencing 50 POP-7 60 96 384 700
Standard sequencing 50 POP-7 120 48 192 850
Long sequencing 80 POP-4 210 24 96 700

139DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Long sequencing 80 POP-7 170 32 128 950
‡ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).
Table 17 3130/3130xl instrument run modules (continued)
Run Module
Array Length
(cm)
Polymer
Run Time
(minutes)
Throughput (samples/day)
Read
Length
‡
(bases)
3130
Analyzer
3130xl
Analyzer
Table 18 3100 instrument run modules
Run Module
Array Length
(cm)
Polymer
Run Time
(minutes)
Throughput
(samples
/day)
Read Length (bases)
ABI
Basecaller
‡
KB
Basecaller
§
Ultra rapid sequencing 36 POP-4 40 576 500 400
Rapid sequencing 36 POP-6 60 384 500 500
Standard sequencing 50 POP-4 100 224 NA 600
Standard sequencing 50 POP-6 150 144 650 600
Long read sequencing 80 POP-4 210 96 950 700
‡ Read length using the ABI basecaller with 98.5% basecalling accuracy.
§ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).
Table 19 3100-Avant instrument run modules
Run Module
Array Length
(cm)
Polymer
Run Time
(minutes)
Throughput
(samples
/day)
Read Length (bases)
ABI
Basecaller
‡
KB
Basecaller
§
Ultra rapid sequencing 36 POP-4 40 144 500 400
Rapid sequencing 36 POP-6 60 96 500 500
Standard sequencing 50 POP-4 100 53 NA 600
Standard sequencing 50 POP-6 150 36 650 600
Long read sequencing 80 POP-4 210 24 950 700
‡ Read length using the ABI basecaller with 98.5% basecalling accuracy.
§ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).

140 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Run Modules for BigDye
®
XTerminator
™
Purified Samples
Applied Biosystems provides BigDye XTerminator Purification Kit run modules for
the 3730/3730xl, 3130/3130xl, and 3100/3100-Avant Data Collection Software v2.0
or greater. Use these run modules when you run a plate containing BigDye
XTerminator reagents. The modules adjust the sample injection height to prevent
injection failures
These run modules can be downloaded from the Applied Biosystems web site. Refer
to Appendix A in the BigDye
®
XTerminator Purification Kit Protocol (PN 4374408)
for instructions on installing these run modules.
Note: If you transfer the supernatant to a clean plate after centrifuging, refer to
Tables 16 through 20 for the appropriate run modules for your instrument.
Table 20 310 instrument run modules
Run Module
Capillary
Length (cm)
Polymer
Run Time
(minutes)
Throughput
(samples
/day)
Read Length (bases)
ABI
Basecaller
‡
KB
Basecaller
§
Standard sequencing 47 POP-4 50 27 525 650
Rapid sequencing 47 POP-4 38 37 425 600
Rapid sequencing 47 POP-6 60 24 400 500
Standard sequencing 61 POP-6 180 8 600 700
‡ Read length using the ABI basecaller with 98.5% basecalling accuracy.
§ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).
Table 21 BigDye
®
XTerminator
™
Purification Kit run modules
Run Module
Capillary
Length (cm)
Polymer
‡
Read Length
§
(bases)
3730/3730xl DNA Analyzer
BDx_XLRSeq50_POP7 50 POP-7
™
900
BDx_LongSeq50_POP7 850
BDx_FastSeq50_POP7 700
BDx_StdSeq36_POP7 36 POP-7
™
700
BDx_RapidSeq36_POP7 550
Applied Biosystems 3100/3100-Avant, 3130/3130xl Genetic Analyzer
BDx_LongSeq80_POP7 80 POP-7
™
950
BDx_LongSeq80_POP4 POP-4
™
700

141DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Modifying Run Modules
The default run modules provided by Applied Biosystems are optimized for many
applications. If you choose to modify run modules, you can use this section for
guidelines on modifying electrokinetic injection and electrophoresis conditions.
For More Information
For more information on modifying run modules, refer to your instrument user
guide:
• Applied Biosystems 3730/3730xl DNA Analyzer Getting Started Guide
(PN 4359476)
• Applied Biosystems 3130/3130xl Genetic Analyzers Getting Started Guide
(PN 4352715)
• ABI P
RISM
®
3100/3100-Avant Genetic Analyzers User Guide (PN 4347102)
• ABI P
RISM
®
310 Genetic Analyzer User Guide (PN 4317588)
Modifying
Electrokinetic
Injection
The best way to control the amount of fluorescence signal generated during a run is
to optimize the amount of DNA template that you use in your sequencing reactions
(see page 63). However, if you consistently observe that the signal is too strong or too
weak or that the resolution is poor, you may modify the injection parameters. The
goal is to inject sufficient DNA to yield peaks of adequate height (that is, data with a
good signal-to-noise ratio) while maintaining resolution and read length.
BDx_StdSeq50_POP7 50 POP-7
™
850
BDx_StdSeq50_POP6 POP-6
™
600
BDx_StdSeq50_POP4 POP-4
™
600
BDx_FastSeq50_POP7 POP-7
™
700
BDx_RapidSeq36_POP7 36 POP-7
™
600
BDx_RapidSeq36_POP6 POP-6
™
500
BDx_UltraSeq36_POP7 POP-7
™
500
BDx_UltraSeq36_POP4 POP-4
™
400
‡ POP-7 can only be used on 3730/3730xl and 3130/3130xl instruments.
§ Read length using the KB basecaller with QVs of 20 (99% basecalling accuracy).
Table 21 BigDye
®
XTerminator
™
Purification Kit run modules (continued)
Run Module
Capillary
Length (cm)
Polymer
‡
Read Length
§
(bases)

142 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Modifying the injection parameters should be viewed as a temporary fix. You should
perform further troubleshooting to determine the root cause of the problem, in order
to extend the lifetime of the capillary array and other system components.
IMPORTANT! Modifying electrokinetic injection can greatly affect data quality and
run-to-run reproducibility.
Note: For information on setting electrokinetic injection values, refer to your
instrument user guide.
Reducing Signal Strength
If the signal is too strong:
• Decrease the concentration of DNA fragments in the sample.
• Decrease the injection time.
• Decrease the injection voltage.
Note: Strong signal intensity can also be a symptom of a late start. If you have a late
start, check the DNA concentration and adjust as necessary.
Increasing Signal Strength
If the signal is too weak:
• Increase the concentration of the DNA extension products.
• Increase the injection time to increase the total electrokinetic injection (EKI)
product. However, increased template loads may result in decreased resolution.
• Reduce the amount of salt in the sample.
IMPORTANT! Negative ions (for example, EDTA and acetate) compete with
DNA for injection. To reduce the amount of salt in a sequencing reaction, use
column purification.
Note: Applied Biosystems does not recommend increasing the voltage to increase
signal for runs on multicapillary instruments. Increasing the voltage may reduce
resolution across the capillary array. It is better to adjust the injection time to increase
signal.
Improving Resolution
If the resolution needs to be improved:
• Decrease the injection time. Decreasing the injection time decreases the signal
strength. To compensate for the loss in signal, lower the salt concentration in the
sample or increase the DNA extension product concentration.
• Decrease the run voltage. The rapid protocol is especially sensitive to changes in
the running voltage. A 10% decrease in running voltage (along with a 20%
increase in collection time) results in a marked improvement in resolution. The
standard protocol is much less responsive to decreases in running voltage.
Modifying
Electrophoresis
Conditions
Modifying electrophoresis conditions (run time, run voltage, and run temperature)
can greatly improve data quality, run-to-run reproducibility, and/or throughput. When
selecting values for these parameters, consider the following factors:
• Desired read length

143DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
• Required degree of resolution
Determining the Required Run Time
To ensure that you collect sufficient data to perform analysis, set the electrophoresis
run time approximately 5 to 10 minutes longer than the migration time of the longest
fragment to detect. You can change the data collection time for special requirements.
For example, you can shorten the data collection time if you only need information
about short extension products (for example, in PCR sequencing).
Run Temperature and Run Voltage
Decreased run voltage or temperature decreases the migration rate of fragments.
Longer run times are required to collect the same size fragments as in standard
conditions. However, the basecallers provided with the Sequencing Analysis
Software have not been optimized to analyze under some modified run conditions;
therefore, the basecallers may not estimate the spacing values successfully.
Increased run voltage or temperature increases migration rates, allowing for shorter
run times, but decreased resolution.
Defining the Analysis Protocol
Analysis protocol are shared between Data Collection Software and any of the
following: Sequencing Analysis Software, SeqScape Software, MicroSeq ID
Analysis Software. (Variant Reporter Software also contains analysis protocols but it
does not interact with Data Collection Software.)
Note: On 310 instruments, create the analysis protocol in Sequencing Analysis
Software using the Analysis Protocol Manager.
Analysis Protocol
Contents
An analysis protocol contains the settings necessary to determine the sequence and to
perform post-processing of the sequence:
• General settings – The name, description of the analysis protocol, and the
sequence file formats to be generated (page 143).
• Basecalling settings – The basecaller, mobility file, analysis stop point, and
other options used by the basecaller (page 144).
• Mixed bases settings (optional) – Setting for identifying mixed bases when two
peaks are found at the same position. Definition for the percent value of the
second highest to the highest peak (page 149).
• Clear range setting – Setting for trimming the low quality sequences that are
typically at the 5′ and 3′ ends. The trimming can be based on base positions,
sample quality values, and/or number of ambiguous bases (Ns) (page 150).
General Settings
Protocol Name
When you name your analysis protocol, assign a name that can help you distinguish
between your analysis protocols.

144 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Sequence File Formats
Select a format for the sequence files generated when analysis is completed. You
save these files in the Sample Manager. The files are exported in the same run folder
as the *.ab1 files.
Basecalling Settings
Basecallers
Sequencing Analysis Software v5.x, SeqScape Software v2.x, and MicroSeq ID
Analysis Software include two basecallers: the KB basecaller and the ABI basecaller.
Both basecallers determine the bases of a sequence, but they differ in how they
process the data.
Note: Variant Reporter Software v1.0 includes only the KB basecaller.
IMPORTANT! For bisulfite sequencing samples, you must use the KB basecaller.
Select a basecaller based on your sequencing chemistry and instrument from the
tables in “Run Parameters for Instrument and Analysis Protocols” on page 151.
Table 22 Sequence file format settings
Setting Function
Write .Seq File Select to create a *.seq file for printing the sequence as a text file or for
use in other software.
•Select ABI format to use with Applied Biosystems software.
•Select FASTA for use with other software.
Write Clear
Range in .Seq
File
When Write .Seq File is selected, select to include only the clear range
sequence (see page 150) in the *.seq file.
Write Standard
Chromatogram
Format (.scf)
Select to create a *.scf file to use with other software.
Note: When created, the .scf extension is not appended to the file
name.
Write Phred
(.phd.1) File
Select to create *.phd.1 file to use with other software if you use the
KB basecaller. Contains the bases with quality values for each base.
Table 23 Comparison of the KB and ABI basecallers
Attribute KB Basecaller ABI Basecaller
Functions • Processes raw traces
• Provides processed traces
• Provides pure bases only
or
Provides pure and mixed calls
• Provides quality values (QVs)
• Generates *.phd.1 and *.scf files
• Provides a sample score
• Processes raw traces
• Provides processed traces
• Provides ACGTN calls

145DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
For more information on the KB basecaller, refer to the KB
™
Basecaller v1.4 FAQ:
KB Basecaller Software v1.4 (PN 4362968),
Mobility File
A mobility file contains mobility shift information. The different dyes affect the
electrophoretic mobility of cycle sequencing extension products, especially for
shorter fragments. However, these mobility shifts are consistent for each dye set, and
they can be corrected during analysis. The analysis software compensates for these
mobility differences by applying mobility shifts to the data so that evenly spaced
peaks are presented in the analyzed data. The mobility file also corrects for the
differences between the dye-to-nucleotide relationships in the raw data and the
analyzed data.
Selecting a Mobility File
Select a mobility file that matches the chemistry and basecaller that you are using.
Mobility shifts and mobility file names for the dRhodamine Terminators are similar
to those for the BigDye Terminators, but the dye-set information differs because the
terminators are labeled with different dyes. Make sure that you use the correct
mobility file to prevent miscalling the C and T bases.
Note: If you select the wrong mobility file, you can change analysis settings and
reanalyze the data with the correct mobility file.
Base assignment options Four options available:
• Assigns ACGT and QV to each peak
• Assigns ACGT and QV to each peak;
reassigns Ns to peaks with QVs below a
defined threshold
• Assigns ACGT or a mixed base and QV
to each peak
• Assigns ACGT or a mixed base and QV
to each peak; reassigns Ns to peaks
with QVs below a defined threshold
Assigns Ns to mixed bases
Note: Further processing, either manually
or with additional software, is required to
assign IUB codes to the Ns or pure bases.
Base assignments for
failed samples
‡
Assigns 5 Ns to the entire sample
Analysis report flags the files containing
failed samples
Attempts to call bases
For failed samples, many Ns are assigned
Baseline appearance in
processed data
Less smooth Smoother
Data processing Calls bases and estimates QVs on
Windows operating systems
Calls bases on Windows operating
systems
Data and future support Applied Biosystems 3730/3730xl,
3130/3100xl, 3100/3100-Avant, and 310
instruments
Development ongoing
Applied Biosystems 3730/3730xl,
3130/3100xl, 3100/3100-Avant, and 310
instruments
Development ceased 2003
‡ Failed samples provide no signals and indicate chemistry failure.
Table 23 Comparison of the KB and ABI basecallers (continued)
Attribute KB Basecaller ABI Basecaller

146 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Tables 25 through 29, starting on page 151, list the appropriate mobility files for
each instrument based on the dye chemistry, polymer, and capillary array to be used.
Refer to your instrument user guide for more specific information about choosing a
mobility file.

147DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Mobility File Names
Mobility file names use a combination of characters to indicate the basecaller,
instrument, chemistry, and polymer type.
Figure 32 shows an example of a mobility file name.
Figure 33 Mobility file name, beginning with the basecaller abbreviation
Table 24 Naming conventions for mobility files
Abbreviation For Runs Using...
Basecaller
KB KB basecaller
DP Dye primer chemistry and the ABI basecaller
DT Dye terminator chemistry and the ABI basecaller
Type of Polymer
POP4 POP-4™ polymer
POP5 POP-5™ polymer
POP6 POP-6™ polymer
POP7 POP-7™ polymer
Chemistry
BDTv3
{BDv3}
BigDye
®
Terminators v3.1 and dGTP BigDye
®
Terminators v3.0
{BDv1}
{BD}
BigDye
®
Terminators v1.1 and dGTP BigDye
®
Terminators
{dRhod} dRhodamine Terminator Cycle Sequencing Kits
{-21M13} Dye primer chemistry with the 21 M13 forward primer labeled
{M13Rev} Dye primer chemistry with the M13 reverse primer labeled
KB_3730_POP7_BDTv3.mob
File ExtensionBasecaller Type
Polymer
Instrument Model Dye Chemistry

148 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Profile
The profile selection scales the processed traces for display in the electropherogram
or the analyzed data view.
Ending Base
With reasonable data quality without sequencing artifacts at the 3′ end, the basecaller
can identify the correct stop point.
The ending base options in the analysis protocol allow you to define an ending base
for the basecaller. You may designate one or more endpoints for data analysis. If you
select all options, the ending base is determined based on the value you enter in the
After __ Bases check box
.
Setting Function
True Profile Select to scale the processed traces uniformly. The average height of
peaks in the region of strongest signal is about equal to a fixed value.
The profile of the processed traces is very similar to that of the raw
traces.
Flat Profile Select to scale the processed traces semi-locally. The average height of
peaks in any region is about equal to a fixed value. The profile of the
processed traces is flat on an intermediate scale (greater than about 40
bases).
Note: This option can be applied only to data analyzed with the KB
basecaller. If you use the ABI basecaller, the profile option reverts to the
True Pro fil e.
Setting Function
At PCR Stop
check box
When the check box is selected, the software determines the analysis
endpoint by locating the signal drop that occurs at the end of a short
PCR fragment (the PCR endpoint). If the endpoint peak is not sufficiently
large, the software may not recognize the PCR endpoint.
Note: Noise after the PCR data is considered by the software as signal,
and the software incorrectly calculates the endpoint after the noise.
IMPORTANT! Applied Biosystems recommends selecting this check
box when analyzing short PCR fragments with the KB basecaller.
After __ Ns in
__ bases
check box
‡
‡ To select this option, you must also select Assign ‘N’ for bases with QV < 15 in the Quality
Threshold section (see below). This setting applies only to the KB basecaller.
Sets the analysis endpoint after a certain number of Ns occur within a
certain number of bases (for example, after 5 Ns are detected within a
range of 10 bases).
The window described by in __ bases differs depending on the
basecaller selected:
•KB basecaller
– the window begins at the 51st base.
•ABI basecaller
– the window begins at the 1st base.
After __ Ns
check box
‡
Sets the analysis endpoint after a certain number of Ns occur (for
example, after 20 Ns are detected).
The basecaller starts calling bases from the 5′ end of the sequence and
stops when the specified number of Ns is reached.
After __ Bases
check box
Sets the analysis endpoint after a certain number of bases (for example,
after 800 bases are detected).

149DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Quality Threshold
(KB Basecaller
Only)
The quality threshold settings determine how base assignments are made with the
KB basecaller.
Note the difference in how the KB basecaller and the ABI basecaller make N calls:
• KB basecaller – Assigns Ns for bases below a certain user-defined QV.
• ABI basecaller – Assigns Ns when the basecaller cannot determine whether the
base is an A, C, G, T, or a mixed base.
Mixed Bases
The mixed bases settings define whether to identify the presence of mixed bases and
the threshold for the identification of mixed bases. Mixed bases settings are active
only with the KB basecaller.
Mixed Bases
Settings (KB
Basecaller Only)
Setting Function
Call all bases
and assign QV
With the KB basecaller, assigns a base and QV to every position.
When the resolution of the data is poor, the KB basecaller provides the
best estimate of the basecall and assigns an appropriate QV to help the
user interpret the accuracy of the basecall.
Assign ‘N’ for
bases with QV
< X
With the KB basecaller, assigns Ns for bases with QVs less than the set
point. Useful if the downstream software application that you use to
view the electropherograms cannot display the QV bars assigned by the
KB basecaller.
IMPORTANT! Use this option with caution if you expect the data to
contain mixed bases. Because mixed bases are typically assigned QVs
lower than pure bases (see “Mixed Bases” on page 149), high quality
mixed base calls may be substituted with Ns.
Setting Function
Use Mixed
Base
Identification
‡
‡ When Use Mixed Base Identification is selected, you must unselect N in the Basecalling tab.
Select to assign mixed bases. This setting is required but not sufficient
for the KB basecaller to assign mixed bases.
2nd highest
peak threshold
Enter a threshold for secondary peaks:
• Positions with secondary peaks below the set value are not called as
mixed bases.
• Positions with secondary peaks above the set value may be called as
mixed bases, depending on the KB basecaller evaluation of other
settings, such as noise level in the sample and peak width.
Note: Do not enter a value less than 15%.

150 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Clear Range
The clear range is the region of sequence that remains after excluding the low-quality
or error-prone sequence at the 5′ and 3′ ends. In the Electropherogram and Sequence
views, the excluded data is displayed in gray. The bases outside the clear range
cannot be edited.
Clear Range
Settings
You can choose one or more methods to set the clear range. If you apply multiple
methods, the methods are applied in order to create a sequence with the smallest
clear range.
Setting Function
Use clear
range
minimum and
maximum
Select to set the clear range based on specified starting and ending
base. Ending base can be specified as a numeric value (for example 500
bases after the start) or as a number of bases to trim from the 3′ end.
Use quality
values
Select to set the clear range based on the number of bases in a window
with a specified quality value.
For example, remove bases from the ends until fewer than 4 bases out
of 20 have QVs <20.
Use
identification
of N calls
Select to set the clear range based on the number of bases in a window
with a specified number of Ns.
For example, remove bases from the ends until there are fewer than 4
Ns out of 20 bases.

151DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Run Parameters for Instrument and Analysis Protocols
Use the tables in this section to identify the appropriate Data Collection Software
parameters for the instrument and analysis protocols.
• Table 25, “3730/3730xl instrument run parameters,” on page 151
• Table 26, “3130/3130xl instrument run parameters,” on page 152
• Table 27, “3100 instrument run parameters,” on page 153
• Table 28, “3100-Avant instrument run parameters,” on page 154
• Table 29, “310 instrument run parameters,” on page 155
Table 25 3730/3730xl instrument run parameters
DNA Sequencing Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run Module Basecaller File (.bcp) Mobility File (.mob)
‡
KB Basecaller
BigDye Terminator v1.1 and
dGTP BigDye Terminator
E POP-7 all lengths all types
KB KB_3730_POP7_BDTv1
BigDye Terminator v3.1 and
dGTP BigDye Terminator v3.0
Z POP-7 all lengths all types
KB KB_3730_POP7_BDTv3
ABI Basecaller
BigDye Terminator v1.1 and
dGTP BigDye Terminator v3.0
E POP-7 36 Rapid
Basecaller-3730POP7RR DT3730POP7{BD}
36 Standard
Basecaller-3730POP7SR
50 Long
Basecaller-3730POP7LR
BigDye Terminator v3.1 and
dGTP BigDye Terminator v3.0
Z POP-7 36 Rapid
Basecaller-3730POP7RR DT3730POP7{BDv3}
36 Standard
Basecaller-3730POP7SR
50 Long
Basecaller-3730POP7LR
‡ Mobility files are not available for running dRhodamine terminator chemistry or BigDye primer chemistry on 3730/3730xl
instruments.

152 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
Table 26 3130/3130xl instrument run parameters
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run
Module
Basecaller File (.bcp) Mobility File (.mob)
KB Basecaller
BigDye
®
Terminator v1.1 and
dGTP BigDye
Terminator
E POP-4 36 Ultra rapid
KB KB_3130_POP4_BDTv1
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3130_POP6_BDTv1
50 Standard
POP-7 36 Ultra rapid
KB KB_3130_POP7_BDTv1
50 Fast
80 Long
36 Rapid
50 Standard
BigDye
®
Terminator
v3.1 and dGTP
BigDye Terminator
v3.0
Z POP-4 36 Ultra rapid
KB KB_3130_POP4_BDTv3
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3130_POP6_BDTv3
50 Standard
POP-7 36 Ultra rapid
KB KB_3130_POP7_BDTv3
50 Fast
80 Long
36 Rapid
50 Standard
ABI Basecaller
BigDye Terminator
v1.1 and dGTP
BigDye Terminator
E POP-4 36 Ultra rapid
Basecaller-3130POP4UR DT3100POP4LR{BD}v1
36 Rapid
Basecaller-3130POP4UR DT3100POP6{BD}v1
POP-6 50 Standard
Basecaller-3130POP6SR
BigDye Terminator
v3.1 and dGTP
BigDye Terminator
v3.0
Z POP-4 36 Ultra rapid
Basecaller-3130POP4UR DT3100POP4{BDv3}v1
80 Long
Basecaller-3130POP4_80cmv3
POP-6 36 Rapid
Basecaller-3130POP6RRv2 DT3100POP6{BDv3}v1

153DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
dRhodamine
Terminator
E POP-4 36 Ultra rapid
Basecaller-3130POP4UR DT3100POP4{dRhod}v2
POP-6 36 Rapid
Basecaller-3130POP6RRv2 DT3100POP6{dRhod}v2
50 Standard
Basecaller-3130POP6SR
BigDye Primer E POP-6 36 Rapid
Basecaller-3130POP6RRv2 DP3130POP6{BD-21M13}v1
DP3130POP6{BD-21M13Rev}v1
50 Standard
Basecaller-3130POP6SR
Table 26 3130/3130xl instrument run parameters (continued)
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run
Module
Basecaller File (.bcp) Mobility File (.mob)
Table 27 3100 instrument run parameters
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run Module Basecaller File (.bcp) Mobility File (.mob)
KB Basecaller
BigDye
®
Terminator v1.1
and dGTP BigDye
Terminator
E POP-4 36 Ultra rapid
KB KB_3100_POP4_BDTv1
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3100_POP6_BDTv1
50 Standard
BigDye Terminator
v3.1 and dGTP
BigDye Terminator
v3.0
Z POP-4 36 Ultra rapid
KB KB_3100_POP4_BDTv3
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3100_POP6_BDTv3
50 Standard
ABI Basecaller
BigDye Terminator
v1.1 and GTP
BigDye Terminator
E POP-4 36 Ultra rapid
Basecaller-3100POP4UR DT3100POP4LR{BD}v1
POP-6 36 Rapid
Basecaller-3100POP6RRv2 DT3100POP6{BD}v2
50 Standard
Basecaller-3100POP6SR
BigDye Terminator
v3.1 and GTP
BIgDye Terminator
v3.0
Z POP-4 36 Ultra rapid
Basecaller-3100POP4UR DT3100POP4{BDv3}v1
80 Long
Basecaller-3100POP4_80cmv3
POP-6 36 Rapid
Basecaller-3100POP6RRv2 DT3100POP6{BDv3}v1
50 Standard
Basecaller-3100POP6SR

154 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
dRhodamine
Terminator
E POP-4 36 Ultra rapid
Basecaller-3100POP4UR DT3100POP4{dRhod}v2
POP-6 36 Rapid
Basecaller-3100POP6RRv2 DT3100POP6{dRhod}v2
50 Standard
Basecaller-3100POP6SR
BigDye Primer E POP-6 36 Rapid
Basecaller-3100POP6RRv2 DP3100POP6{BD-21M13}v1
DP3100POP6{BD-M13Rev}v1
50 Standard
Basecaller-3100POP6SR
Table 27 3100 instrument run parameters (continued)
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run Module Basecaller File (.bcp) Mobility File (.mob)
Table 28 3100-Avant instrument run parameters
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run
Module
Basecaller File (.bcp) Mobility File (.mob)
KB Basecaller
BigDye
®
Terminator
v1.1 and dGTP
BigDye Terminator
EPOP-4 36 Ultra rapid
KB KB_3100_POP4_BDTv1
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3100_POP6_BDTv1
50 Standard
BigDye
®
Terminator
v3.1 and dGTP
BigDye Terminator
v3.0
ZPOP-4 36 Ultra rapid
KB KB_3100_POP4_BDTv3
50 Standard
80 Long
POP-6 36 Rapid
KB KB_3100_POP6_BDTv3
50 Standard
ABI Basecaller
BigDye Terminator
v1.1 and dGTP
BigDye Terminator
EPOP-4 36 Ultra rapid
Basecaller-3100APOP4UR DT3100POP4LR{BD}v1
POP-6 36 Rapid
Basecaller-3100APOP6RRv2 DT3100POP6{BD}v2
50 Standard
Basecaller-3100APOP6SR
BigDye Terminator
v3.1 and dGTP
BigDye Terminator
v3.0
ZPOP-4 36 Ultra rapid
Basecaller-3100APOP4UR DT3100POP4{BDv3}v1
80 Long
Basecaller-3100APOP4_80cmv3
POP-6 36 Rapid
Basecaller-3100APOP6RRv2 DT3100POP6{BDv3}v1

155DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
dRhodamine
Terminator
EPOP-4 36 Ultra rapid
Basecaller-3100APOP4UR DT3100POP4{dRhod}v2
POP-6 36 Rapid
Basecaller-3100APOP6RRv2 DT3100POP6{dRhod}v2
50 Standard
Basecaller-3100APOP6SR
BigDye Primer E POP-6 36 Rapid
Basecaller-3100APOP6RRv2 DP3100POP6{BD-21M13}v1
DP3100POP6{BD-M13Rev}v1
50 Standard
Basecaller-3100APOP6SR
Table 28 3100-Avant instrument run parameters (continued)
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Run
Module
Basecaller File (.bcp) Mobility File (.mob)
Table 29 310 instrument run parameters
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Basecaller File (.bcp) Mobility File (.mob)
KB Basecaller
BigDye
®
Terminator v1.1
and dGTP BigDye
®
Terminator
EPOP-4 47
KB KB_310_POP4_BDTv1_36Rapid
KB_310_POP4_BDTv1_36Std
POP-6 47
KB KB_310_POP6_BDTv1_36Rapid
POP-6 61
KB KB_310_POP6_BDTv1_50Std
BigDye
®
Terminator v3.1
and dGTP BigDye
Terminator v3.0
‡
EPOP-4 47
KB KB_310_POP4_BDTv3_36Rapid
KB_310_POP4_BDTv3_36Std
POP-6 47
KB KB_310_POP6_BDTv3_36Rapid
POP-6 61
KB KB_310_POP6_BDTv3_50Std
ABI Basecaller
BigDye
®
Terminator v1.1
and dGTP BigDye
®
Terminator
EPOP-4 47
Basecaller-310POP4 DT310POP4{BD}v2
POP-6 47
Basecaller-310POP6 DT310POP6{BD}
POP-6 61
Basecaller-310POP6 DT310POP6{BD-LR}v3
BigDye
®
Terminator v3.1
and dGTP BigDye
Terminator v3.0
ZPOP-4 47
Basecaller-310POP4 DT310POP4{BDv3}v2
POP-6 47
Basecaller-310POP6 DT310POP6{BDv3}v2
DT310POP6{BDv3}v2
POP-6 61
dRhodamine Terminator E POP-4 47
Basecaller-310POP4 DT310POP4{dRhod}v1
POP-6 47
Basecaller-310POP6 DT310POP6{dRhod}v2

156 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 6 Capillary Electrophoresis
BigDye
®
Primer E POP-4 47
Basecaller-310POP4 DP310POP4{BD-21M13}v1
DP310POP4{M13Rev}v1
POP-6 47
Basecaller-310POP6 DP310POP6{BD-21M13}v1
DP310POP6{M13Rev}v1
POP-6 61
‡ For instructions on running BigDye terminators v3.1 and dGTP BigDye terminators v3.0 on 310 instruments, refer to the
310 instrument user guide.
Table 29 310 instrument run parameters (continued)
DNA Sequencing
Chemistry
Dye
Set
Polymer
Type
Capillary
Array
Length
(cm)
Basecaller File (.bcp) Mobility File (.mob)

157DNA Sequencing by Capillary Electrophoresis Chemistry Guide
7
Data Analysis 7
This chapter covers:
Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .158
Reviewing Data with Sequence Scanner Software . . . . . . . . . . . . . . . . . . . . . . . . .164
Analyzing Data with Sequencing Analysis Software . . . . . . . . . . . . . . . . . . . . . . .170
Analyzing Data with Variant Reporter™ Software. . . . . . . . . . . . . . . . . . . . . . . . .175
Analyzing Data with SeqScape® Software. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .181
Analyzing Data with MicroSeq® ID Analysis Software . . . . . . . . . . . . . . . . . . . .188

158 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Overview
This chapter provides an overview of data analysis using Applied Biosystems
software for DNA sequencing. The information in this chapter is a high-level view of
the software and does not contain detailed instructions for using the software. For
detailed instructions, please refer to the appropriate user guide and/or online help.
Workflow
DNA Template Preparation (Chapter 3)
Cycle Sequencing (Chapter 4)
Extension Product Purification (Chapter 5)
Capillary Electrophoresis (Chapter 6)
Data Analysis
1. Choose software for your application.
2. Apply analysis protocols, including:
– Basecaller
– Mobility file
3. Run analysis.
4. Review the data.
Applied Biosystems software:
• Sequence Scanner Software
• Sequencing Analysis Software
• Variant Reporter
TM
Software
•SeqScape
®
Software
•MicroSeq
®
ID Analysis Software
Data Analysis Output:
Analyzed project in Variant Reporter
Software
Analyzed project in
SeqScape Software
Analyzed sample file in
Sequencing Analysis
Software

159DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Analysis Software
The following analysis software is available from Applied Biosystems at
www.appliedbiosytems.com:
Choosing Analysis Software by Application
Answer the following questions to help you to choose the most appropriate package
for your lab or review “Choosing Analysis Software by Available Features” on
page 162.
1. What applications are performed in your lab with your genetic analyzer?
Use Table 31 below to select an analysis software package.
If the table recommends more than one type of software, go to 2.
Table 30 Analysis software available from Applied Biosystems
Product Suggested Use
Sequence Scanner Software Viewing or editing traces, evaluating trace quality, making
trace QC reports
Sequencing Analysis
Software
Viewing or editing traces, evaluating trace quality, making
trace QC reports, reanalyzing traces
Variant Reporter™ Software Detecting mutations, discovering and validating SNPs
SeqScape
®
Software Detecting mutations, discovering and validating SNPs,
comparing sequences, typing
MicroSeq
®
ID Analysis
Software
With the MicroSeq ID Kit, identifying bacteria and fungi
Table 31 Analysis software recommendations, by application
Sequencing Application
Sequencing
Analysis
Software
Variant
Reporter
™
Software
SeqScape
®
Software
Sequence quality control Yes Yes No
BAC end sequencing Yes No No
Checking clone constructs Not
Recommended
Ye s Ye s
De novo sequencing Yes No No
Multi Locus Sequence Typing (MLST) Not
Recommended
No Yes
HLA typing Not
Recommended
No Yes
Methylation analysis using bisulfite DNA conversion Not
Recommended
Ye s Ye s
Mitochondrial DNA (mtDNA) sequencing No Yes Yes

160 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
2. Are 21CFR part 11 compliance features required in your lab?
and/or
Do you use BigDye Primer Chemistry?
• If the answer is yes to either question, choose SeqScape Software.
SeqScape Software includes 21CFR part 11 compliance features such as
User Access Protection, Audit Trail and Electronic Signature and is also
the only analysis software that can analyze data generated using BigDye
Primer Chemistry.
• If no, continue to 3.
3. Will you be analyzing projects without a reference sequence?
and/or
Do you need to analyze large projects (>500 traces)?
• If the answer is yes to either question, choose Variant Reporter Software.
This package can use higher quality traces to build its own reference,
whereas SeqScape Software requires a reference file.
Variant Reporter Software is specifically designed to handle up to 5000
traces in only one project. Basecalling, the most time-consuming part of
the analysis, can be skipped if data are pre-basecalled by Sequencing
Analysis Software.
• If no, continue to 4.
4. If none of the above questions have helped you decide, you can:
• Examine Table 32 for more information.
• Download a demo version of either SeqScape Software or Variant Reporter
Software from www.appliedbiosystems.com.
To learn more, you can register for a web-based training course at
https://learn.appliedbiosystems.com/sequencing.
• Contact your Applied Biosystems sales representative or field application
specialist.
Resequencing – substitutions (SNPs), heterozygous
mutations, insertions and deletions
Not
Recommended
Ye s Ye s
Virus subtyping Not
Recommended
No Yes
Table 31 Analysis software recommendations, by application (continued)
Sequencing Application
Sequencing
Analysis
Software
Variant
Reporter
™
Software
SeqScape
®
Software

161DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Table 32 Features in Applied Biosystems analysis software
Software Features and Functions
Sequencing Analysis
Software
With Sequencing Analysis Software you can:
• Perform basecalling and trim low quality bases at the 5′ and 3′ ends. With the KB
™
basecaller, you can also:
– Identify pure and mixed bases
– Assign a quality value for each base
– Replace Ns for bases with quality values below a set threshold
– Detect failed sequence samples
– Calculate quality values (QV) for each base
• Reanalyze the data by modifying analysis settings
• Review data for troubleshooting using metrics provided in the Analysis Report
• Export analyzed sequences to formats including *.seq, *.phd1 and *.scf to integrate
with downstream workflows
• Create an audit trail to track all changes to bases and analysis settings
Variant Reporter
™
Software With Variant Reporter Software, you can:
• Analyze sequence data and generate an accurate consensus sequence for each
specimen using bidirectional sequencing coverage
• Call and edit bases using the improved algorithms and additional quality metrics in
KB™ Basecaller v1.4
• Align the consensus sequence to identify variants
• Import a reference sequence from a public database such as the National Center for
Biotechnology Information (NCBI)
• Customize the reference sequence by creating unique sequence layers composed
of different features, such as exons, introns, and untranslated regions (UTRs)
• Set quality control metrics to filter out low quality data, ensuring higher confidence
results and reducing manual review time
• Archive projects with sample files and all associated reference data (analysis
settings, reference, etc.) for data sharing purposes
SeqScape
®
Software With SeqScape Software, you can:
• Analyze sequence data and generate an accurate consensus sequence for each
specimen using bidirectional sequencing coverage
• Call bases using the improved algorithms and additional quality metrics in KB™
Basecaller v1.4
• Import a reference sequence from a public database such as the National Center for
Biotechnology Information (NCBI)
• Compare sequence data to the known reference sequence to identify variants or to
a library of sequences to identify the perfect match
• Display amino acid variants in the consensus sequence
MicroSeq
®
ID Analysis
Software
With MicroSeq ID Analysis software, you can:
• Compare sequence data from unknown bacterial or fungal species to sequences of
known bacteria or fungi stored in a library
• Generate a final identification list of organisms that are the closest matches to the
unknown, based on a percent similarity that reflects how closely the unknown
isolate matches the library sequence

162 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Choosing Analysis Software by Available Features
This section describes the different analysis tasks that can be performed by Applied
Biosystems analysis software for capillary electrophoresis sequencing instruments.
• Basecalling – Translates the collected color-data images into the corresponding
nucleotide basecalls.
• Autoanalysis – Enables automatic analysis after data collection from the
Genetic Analyzer.
• Sequence alignment with reference – Aligns sample files (traces) to a user-
supplied reference DNA sequence.
• Sequence alignment, no reference – Aligns sample files (traces) to each other
to create a reference DNA sequence.
• Mutation detection – Compares the sample files (traces) to a reference DNA
and detects nucleotide substitutions, insertions, deletions, and heterozygous
insertion/deletions.
• Typing – Compares the sample files (traces) to a group of known reference
DNA sequences and determines the best match.
• Audit and e-signature features – Assists with 21 CFR Part 11 compliance,
providing audit trail, access control, and e-signature features.
Note: Table 33 lists the tasks that each analysis software performs.
Sequence Scanner Software With Sequence Scanner Software, you can:
• View, edit, print, and export sequence data
• Align analyzed peaks to raw data peaks
• Map traces to plate well positions to visualize quality patterns
• Generate and export graphical reports
• Export sequence data to file formats including *.fsta, *.seq, *.phd1 and *.scf
Table 32 Features in Applied Biosystems analysis software (continued)
Software Features and Functions
Table 33 Tasks performed by Applied Biosystems analysis software
Feature
Software Package
Sequence
Scanner
Software
Sequencing
Analysis
Software
Variant
Reporter™
Software
SeqScape
®
Software
MicroSeq
®
ID
Analysis
Software
Basecalling No Yes Yes
‡
Yes
‡
Ye s
Autoanalysis No Yes No Yes Yes
Sequence alignment with
reference
No No Yes Yes No
Sequence alignment, no
reference
No No Yes No No
Mutation detection No No Yes Yes No

163DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Data Quality
Signal-to-noise ratios and variation in peak heights heavily influence the accuracy of
heterozygote detection. If the data noise level is high, heterozygote detection
algorithms are likely to detect many false positives that you will need to review
manually.
The degree of variation in peak heights depends on the sequencing chemistry used.
Because BigDye
®
Terminator chemistry produces very even signal intensities, the
number of false negatives is reduced. Sequencing using alternative dye terminator
chemistries may produce data with uneven peak heights, which may result in the
failure to detect many true heterozygote locations.
Well-defined peak resolution, uniform peak spacing, and high signal-to-noise ratios
characterize good quality sequencing data. These characteristics enable more
accurate automated mixed-base identification, which saves time that might otherwise
be required for manual sequence review and editing.
Ty p i n g
§
No No No Yes Yes
Auditing and electronic
signature features (21 CFR
Part 11)
No Ye s N o Yes Yes
‡ For reanalysis
§ With sequence library
Table 33 Tasks performed by Applied Biosystems analysis software (continued)
Feature
Software Package
Sequence
Scanner
Software
Sequencing
Analysis
Software
Variant
Reporter™
Software
SeqScape
®
Software
MicroSeq
®
ID
Analysis
Software

164 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Reviewing Data with Sequence Scanner Software
Overview and
Applications
Sequence Scanner Software allows you to view, edit, print, and export data generated
using Applied Biosystems genetic analyzer instruments after data has been processed
by Sequencing Analysis Software.
Software
Workflow
1. Perform basecalling using Sequencing Analysis Software.
2. Import data into Sequence Scanner Software.
3. Review the results.
4. Export and print results and reports.
For More
Information
See Chapter 8 for information on troubleshooting sequencing data.
Tips and Hints
• Sequence Scanner Software can be downloaded for free from the Applied
Biosystems website at www.appliedbiosystems.com/sequencescanner.
• Exporting results of quality analysis as an Excel file or importing into a custom
database is a good way to monitor system variables over time.
This section contains some frequently asked questions about Sequence Scanner
Software.
• “How do I review and edit bases?” on page 164
• “How can I use the reports to review the data?” on page 166
• “How do I export traces or sequences?” on page 169
How do I review and edit bases?
1. Use the Sequence View to identify bases with low quality values.
In the figure below:
• Base 71 is red and displays a mixed base call of Y with a low quality value
(QV) of 2 (shown in the tool tip).
• Base 75 is also red, with a mixed base call of Y.

165DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
2. Click the base to select it, then click the Analyzed+Raw tab.
The yellow highlight indicates the selected base in the sequence and in the
Analyzed data pane (at the bottom of the window).
The arrow shows the corresponding base in the raw data. (The raw data has
different mobility than the analyzed data). In the Analyzed data pane it is
apparent that there is an unambiguous T base at position 71 and a C base peak at
position 75.
The mixed base call is caused by an elevated baseline due to poor resolution of
the CTCC sequence.
3. Type over the nucleotide sequence to change the mixed base calls:
• At base 71, change the mixed base call to T.
• At base 75, change the mixed base call to C.
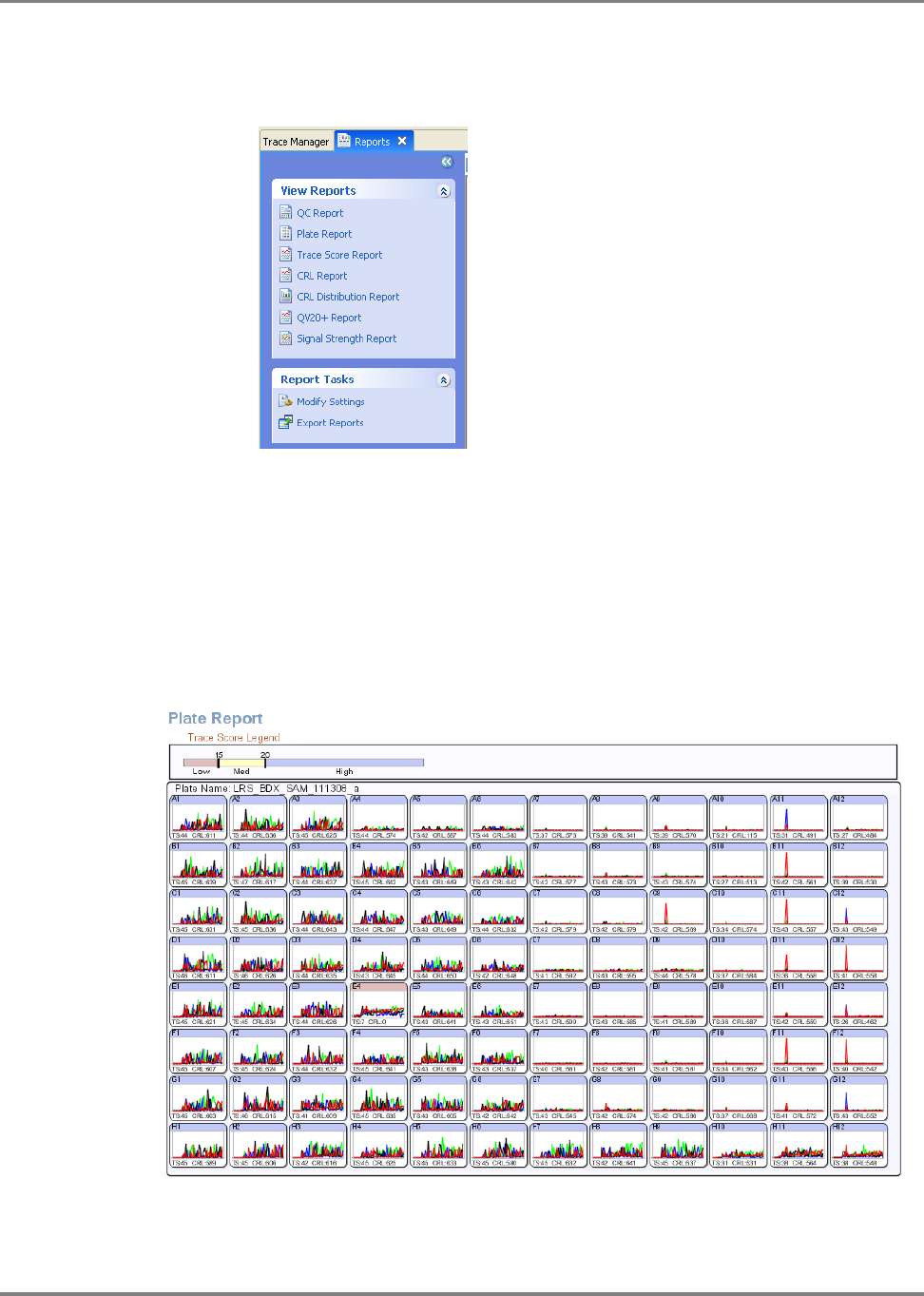
166 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How can I use the reports to review the data?
1. Click the Reports tab to view the View Reports menu.
2. Click Plate Report to view the Plate Report.
The Plate Report gives an at-a-glance overview of the data quality of the plate
that was processed for sequencing. The prominent peak seen in some wells on
the right side of the plate indicates the presence of a strong dye blob; these
samples were not purified with sufficient BigDye Exterminator reagent prior to
electrophoresis.
Note that sample E4 (capillary 24) shows a red header bar; this indicates a failed
result.
Double-click any of the wells to view the corresponding trace.

167DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
3. Click Signal Strength Report.
The Signal Strength Report shows the average intensity of the raw signal for the
four bases in each sample.
Note that there is considerable variation in signal strength from sample to
sample. This is typical in most electrokinetic injections. The signal strength is
not a suitable metric for overall data quality. However, if signal strength is below
200 rfu for a number of samples the data quality of the traces may appear poor
and the validity of the data should be carefully examined. Re-injecting the
samples using longer injection times may boost the signal strength.
Click any of the data points in the graph to open the corresponding trace.

168 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
4. Use the Contiguous Read Length Report (CRL) to easily identify the longest
uninterrupted stretch of bases with a set quality value (e.g. QV20).
In the report below, note the drop-out in capillary 24; this indicates a failed
sample, probably due to a clogged capillary.
A similar report is the QV20 report that scores the longest sequence read with a
given quality value.
Click any of the columns in the graph to open the corresponding trace.

169DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
5. Use the Thumbnail view to display a graphical view of the traces.
In the figure below, the peak in sample 32 indicates a dye blob. Sample 24 is the
sample with the clogged capillary.
Double-click any of the thumbnails to open the corresponding trace.
How do I export traces or sequences?
1. In the Trace Manager tab, click Export Traces to open the Export Traces dialog
box.
2. Check the options for the sequence and the file format, then click OK.
3. (Optional) For sequences in FASTA format, you can use the BLAST database
search to identify the sequence.
A BLAST server is located at: http://blast.ncbi.nlm.nih.gov/Blast.cgi.
Sample 32
Sample 24

170 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Analyzing Data with Sequencing Analysis Software
Overview and
Applications
Sequencing Analysis Software is a data review tool designed to basecall, trim,
display, edit, and print data from all Applied Biosystems capillary electrophoresis
DNA sequencing instruments.
Software
Workflow
1. Import sample files.
2. Define the analysis protocol.
3. Run the analysis.
4. Review the results.
5. If needed, edit the bases.
6. If needed, reanalyze the data.
7. Generate the analysis report and/or print traces.
For More
Information
See Chapter 8 for information on troubleshooting sequencing data.
Refer to the following documents for more information about Sequencing Analysis
Software:
• Sequencing Analysis Software v5.2 User Bulletin (PN 4358355)
• Applied Biosystems DNA Sequencing Analysis Software Version 5.1 for
Windows
®
XP and 2000 Platforms User Guide (PN 4346366)
To obtain the latest software updates and patches, go to
www.appliedbiosystems.com, then click the link for Support.
Tips and Hints
Performing analysis with Sequencing Analysis Software can reduce processing time
in Variant Reporter Software.
This section contains some frequently asked questions about Sequencing Analysis
Software.
• “Why do I see Ns in samples analyzed by the KB™ Basecaller Software?” on
page 171
• “Why are the quality value bars displayed in gray?” on page 171
• “How can I adjust the y-axis so that all traces have the same scale?” on page 171
• “What are the differences between quality values of mixed bases and pure
bases?” on page 172
• “How do I find the QV chart?” on page 172
• “How do I select what I want to print?” on page 173
• “How can I increase the number of traces displayed in the Sample View
window?” on page 174
• “Why does the spacing value sometimes appear in red and have a negative
value?” on page 174

171DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Why do I see Ns in samples analyzed by the KB™ Basecaller Software?
When using the KB Basecaller, you see the sequence “NNNNN” when the sample
failed analysis. Omit this file from further analysis. The Analysis Report in
Sequencing Analysis Software also flags these files.
In addition to pure and mixed bases with QV bars, you may also see N’s and gray QV
bars when you choose to reassign Ns to all bases with less than the user-specified QV
threshold. This option is present to allow those who analyze data with the KB
Basecaller but share data with others who do not have software to display the quality
values. This allows you to take advantage of the longer read length and more accurate
basecalling provided by the KB Basecaller while still viewing data with software that
does not display QVs.
Why are the quality value bars displayed in gray?
A quality value is assigned to a specific basecall. If you alter the basecall, or reassign
the bases as Ns for bases below a certain QV, the QV bar is not applicable to the new
basecall and it is displayed as a gray bar. The base is shown in lower-case text
indicating the basecall has been altered.
How can I adjust the y-axis so that all traces have the same scale?
1. Select ViewY Axis ScaleScale To… to open the Set Y Scale for all
Electropherograms and Raw Data dialog box.
2. Enter the values for the data range for each view.
3. Click Apply to view the data with the new data range. If the range is acceptable
click OK to save the settings.

172 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
What are the differences between quality values of mixed bases and pure bases?
The definition of quality values is the same for pure and mixed bases. In both cases
the probability of error for the associated basecall is 10-q/10. The distribution of
quality values assigned to mixed bases, however, differs dramatically from that for
pure bases. Typically, high quality pure bases will be assigned QVs of 20 or higher.
Good mixed bases, on the other hand, may be assigned quality values as low as 10.
The reason that a high quality mixed base may receive such low QVs is that the
probability of error with more complex signals is higher. Do not discard mixed bases
with QV between 10 and 20. It is a good idea to review them. For mixed bases,
quality values greater than 30 are rare.
How do I find the QV chart?
Select HelpQuality Values Chart.

173DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How do I select what I want to print?
1. Select Tools Options and click the Printing tab.
2. In the Options dialog box, select the print options and the data tabs to print, then
click OK.

174 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How can I increase the number of traces displayed in the Sample View window?
1. Select Analysis Display Settings and click the Data tab.
2. Change the values in the Pane Height, Multiple Samples row for the different
views, then click OK.
• To increase the number of traces displayed, enter smaller values.
• To decrease the number of traces displayed, enter larger values.
Why does the spacing value sometimes appear in red and have a negative value?
• For the ABI Basecaller – When the ABI Basecaller fails to determine a spacing
value for a sample file, it uses a default value of 12.00 for all run conditions.
This number appears in red in the Sample Manager and the Annotation view
displays “–12.00”.
• For the KB Basecaller – When the KB Basecaller fails to determine a spacing
value for a sample file, it uses a default value specific to the particular
instrument/polymer/chemistry/run condition used to generate the sample file.
This number appears in red in the Sample Manager and the Annotation view
displays –1 times this value.

175DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Analyzing Data with Variant Reporter
™
Software
Overview and
Applications
Variant Reporter™ Software is an intuitive resequencing software designed to
accurately discover and validate mutations and SNPs. Variant Reporter Software
provides a task-based guided workflow and analyzes large-volume data faster than
other software.
Variant Reporter Software, with a more intuitive workflow, is designed for:
• Large numbers of samples
• SNP discovery and validation
• Mutation analysis and heterozygote identification
• Sequence confirmation for mutagenesis or clone-construct confirmation studies
Software
Workflow
1. Import and assign traces into amplicons.
2. Define the analysis protocol.
3. (Optional, but recommended) Specify a reference sequence (either a text or
trace file) for the project.
4. Set up the amplicons.
5. Analyze the project.
6. Review variants.
7. Export and print results and/or reports.
For More
Information
See Chapter 8 for information on interpreting and troubleshooting results.
Refer to the Applied Biosystems Variant Reporter
™
Software v1.0 User Guide
(PN 4376590) and Variant Reporter Software online help for more information.
To obtain the latest software updates and patches, go to
www.appliedbiosystems.com, then click the link for Support.
Tips and Hints
This section contains some frequently asked questions about Variant Reporter
Software.
• “How do I align my amplicons to the reference? ” on page 175
• “Why don’t my amplicons align with the reference?” on page 176
• “How do I edit bases?” on page 177
• “How can I save time during the Variant Reporter Software analysis?” on
page 178
• “How do I change the folder where my project data is stored?” on page 178
• “How do I remove or exclude traces from my project? ” on page 180
How do I align my amplicons to the reference?
1. In the Define Reference page, click Amplicon Primers/Known Variants, then
select Add/Edit Primer Sequences to open the Add/Edit Primer Sequences
dialog box.

176 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
2. Click Add, place your cursor in the Amplicon column, then double-click to
enter an amplicon name. (Double-clicking the table activates the edit mode,
allowing you to enter a name manually).
3. To enter a value for the forward and reverse primer sequences from outside
Variant Reporter
Software, copy the value from your source and right-click and
select Paste (or Ctrl+V) into the appropriate column.
4. To align your primer sequences automatically, click Align, then click OK.
Why don’t my amplicons align with the reference?
Amplicon names in the grid must match the amplicon names in the reference.
Unaligned ampliconReference sequence Aligned amplicon

177DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How do I edit bases?
1. Open the Variant Review page.
2. In the Trace Segment tab, select a base in the consensus sequence.
3. Enter the new letter (IUB code) that you want to change the base to, then click
OK at the prompt.
4. To remove a base, select it in the consensus sequence, click Delete, then click
OK.

178 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How can I save time during the Variant Reporter Software analysis?
You can save time if you import pre-basecalled sample files into Variant Reporter
Software.
1. Use Sequencing Analysis protocol templates specific to your instrument, or edit
your existing analysis protocol to match the following:
a. Click the Basecalling tab and select Do not assign Ns to Basecalls.
b. Click the Mixed Bases tab and select Use Mixed Base Identification.
How do I change the folder where my project data is stored?
1. Open the Dashboard, then in the Data Store section, click Manage.
179DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
2. Click Create to select a new location.
3. In the Create Data Store dialog box, enter a name for the Data Store, browse to
where you want the Data Store to reside (the target folder must be empty), then
click OK.
Note: If you want password protection, select Enable Password for Access
before clicking OK.
4. In the Manage Data Store dialog box, the current location of your Data Store is
indicated by bold font. Select the new Data Store location, then click Change
Location.

180 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How do I remove or exclude traces from my project?
1. Open the Import and Manage Traces page.
2. To remove a trace permanently from a project:
a. Select a cell in the Amplicon and Specimen Trace grid. All the traces
represented by the cell fill the Traces in Selected Cell pane.
b. Select the trace(s) you want to remove, right-click the selected trace(s),
then select Remove Selected Traces.
c. Click Ye s when prompted to verify the trace removal. The changes affect
the Amplicon and Specimen Trace Grid and the Amplicon Specimen
Quality Grid.
3. To exclude a trace from a project:
a. Select a cell in the Amplicon and Specimen Trace Grid. All the traces
represented by the cell fill the Traces in Selected Cell pane.
b. Select the traces you want to exclude, right-click the traces, then select
Exclude Selected Traces. (Excluding a trace prevents analysis of the trace
with the project data).
Tips for Verifying Heterozygotes
Two useful ways to verify a heterozygote in a sample are:
• Run the selected chemistry on a reference or a wild-type sample.
• Sequence the opposite strand of the potentially heterozygous sample.
If your application requires close to 100% heterozygote detection, you must run both
samples and compare the sequences and peak heights of both orientations of the
homozygous reference to both orientations of the sample.
In a resequencing study, you obtain the consensus sequence for each donor by
assembling one or more traces for each sequenced region. The consensus-calling
algorithm in Variant Reporter Software improves the accuracy of the consensus
sequence for each donor. It is particularly designed to identify heterozygous bases in
the electropherogram traces. The algorithm also assigns calibrated quality values for
each consensus base to help you review data quality.

181DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Analyzing Data with SeqScape
®
Software
Overview and
Applications
SeqScape
Software is a sequence comparison tool designed for nucleotide and amino
acid variant identification and allele library searching. It is used in resequencing
applications, where the DNA sequence from specific genes or regions from one or
more individuals is compared to a known reference sequence to determine if any
genetic variations are present.
Common goals for resequencing include:
• SNP discovery and validation
• Mutation analysis and heterozygote identification
• Sequence confirmation for mutagenesis or clone-construct confirmation studies
• Identification of genotype, allele, and haplotype from a library of known
sequences
• Pathogen subtyping
• Allele identification
• Sequence confirmation
Software
Workflow
1. Create a project template.
2. Add sequence files to the project.
3. Analyze the data (manually or automatically)
.
4. Review the data using quality values and the Analysis Report.
5. Review the Mutations Report using the Project view and the QC and Mutations
reports.
6. If needed, modify settings or edit data.
7. Export and print results and/or reports.
For More
Information
See Chapter 8 for information on interpreting and troubleshooting results.
Refer to the Applied Biosystems SeqScape
®
Software v2.5 User Guide (PN 4359442)
and online help for more information.
To obtain the latest software updates and patches, go to
www.appliedbiosystems.com, then click the link for Support.
Tips and Hints
This section contains the following tips and hints:
• “How do I set analysis settings?” on page 182
• “How do I set preferences for viewing data for all my projects?” on page 183
• “What is a Reference Data Group?” on page 184
• “How do I create a Reference Data Group?” on page 184
• “Tips for Reviewing Results in the Project View” on page 188
• “Tips for Reviewing Results in the Report View” on page 188
• “Tips for Verifying Heterozygotes” on page 188

182 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
How do I set analysis settings?
You set analysis settings in the Edit Analysis Settings dialog box.
1. Select ToolsSeqScape Manager. In the SeqScape Manager dialog box, click
the Analysis Defaults tab.
2. Click a row in the table for the default settings to change, then click Properties.
The Edit Analysis Settings dialog box opens.
3. Click General to set naming conventions and how modifications made by other
users are monitored.
4. Click Specimen and:
• Set the penalties for the alignment of samples to the reference sequence.
• If you have called the bases in Sequencing Analysis Software, uncheck
Basecall Samples so that SeqScape Software performs only sample
filtering and assembly.

183DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
5. Click Save to close the dialog box and then Close to close the SeqScape
Manager dialog box.
How do I set preferences for viewing data for all my projects?
Set preferences for viewing data in the Edit Display Settings dialog box.
1. Select ToolsSeqScape Manager. In the SeqScape Manager dialog box, click
the Display Settings tab.
2. Click a row in the table for the display settings you want to change, then click
Properties.
The Edit Display Settings dialog box opens.
3. Click Views and make changes to a variety of basic preferences.
In the General View Setting group, click the buttons for the data to display in a
projects.
4. Click Save to close the dialog box and then Close to close the SeqScape
Manager dialog box.

184 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
What is a Reference Data Group?
SeqScape Software compares the consensus segments to the Reference Data Group
(RDG). The Reference Data Group contains the reference sequence and reference-
associated data. The reference sequence is the “backbone” sequence for the project,
consisting of one or more reference segments separated by reference breaks.
The RDG contains gene- and analysis-specific information including:
• Layers, which are units of analysis in any project, and regions of interest (ROIs)
grouped together into layers for display and translation:
– Associated allele libraries
– User-defined styles for identification of variants in the project
• A reference sequence, made up of one or more reference segments, which are
continuous or discontinuous sequences made up of one or more reference
segments.
• One or more reference segments. A reference segment is a portion of the
reference sequence corresponding to a single contiguous DNA sequence. It is
also a region of interest. The reference segment can consist of any of the
following:
– An analyzed sample file
– A text-only, FASTA, or .seq file
– A GenBank format file
How do I create a Reference Data Group?
Create a Reference Data Group in one of two ways.
• Use the RDG Wizard.
or
• Use the SeqScape Software Manager dialog box to open a new RDG.
1. For either method, select ToolsSeqScape Manager. In the SeqScape
Manager dialog box, click the Reference Data Group tab.
2. Click Wizard.
3. Enter a name for the reference data group, select the codon table and click Next.

185DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
4. Add reference segments:
a. Click Add Ref Segment.
b. In the Open File dialog box, locate the file containing the reference
segment, and then click Import.
You can select files in *.txt, *.fsta, *.seq, or GenBank format. You can also
select analyzed sample files or an aligned consensus sequence in FASTA
formats.

186 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
c. Click Next to open the Create ROIs page.
5. In the Create ROIs page, define a region of interest:
• Click and drag to highlight the ROI in the sequence, then click Add ROI.
or
• Enter the first base pair in the Starting with field, the last base pair in the
Ending with field, then click Find. If the ROI is correct, click Add ROI.
Regions of Interest (ROI) define exons, introns, splice junctions, etc.
The ROI (region of interest) can be generated from several different
sources. Depending upon the difficulty of the gene and structure the ROI
can consist of many different layers to define all the areas of interest.
6. Click Next to add layers. For each layer:
a. Enter the name of the layer in the Layer Name field.

187DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
b. Check the check box to the left of each ROI to be included in this layer.
c. Click New Layer.
Create layers to indicate any smaller portions of interest within the main
sequence by highlighting parts of the sequence. These can be color-coded
for quick identification.
7. Click OK.
The new RDG appears in the Reference Data Group list in the SeqScape
Manager dialog box.
8. To view the RDG, in the SeqScape Manager dialog box, click the row for the
RDG in the Reference Data Group tab then click Properties.

188 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
Tips for Reviewing Results in the Project View
• Use the Character/Dots view to easily scan for constant position errors.
• Use tab-jumps to easily scan low quality bases.
• Review the quality values displayed above every basecall.
Tips for Reviewing Results in the Report View
• Use the hyperlinks in the report to navigate between the report and the data.
• Tile the windows to view the report and the data at the same time.
• In the Mutations report, sort the mutations or genotypes according to the QV by
double-clicking the column header.
• In the report, hide or show columns by right-clicking the column header.
IMPORTANT! Make sure you save your project after analyzing and reviewing your
data.
Tips for Verifying Heterozygotes
Two useful ways to verify a heterozygote in a sample are:
• Run the selected chemistry on a reference or a wild-type sample.
• Sequence the opposite strand of the potentially heterozygous sample.
If your application requires close to 100% heterozygote detection, you must run both
samples and compare the sequences and peak heights of both orientations of the
homozygous reference to both orientations of the sample.
In a resequencing study, you obtain the consensus sequence for each donor by
assembling one or more traces for each sequenced region. The consensus-calling
algorithm in SeqScape Software improves the accuracy of the consensus sequence
for each donor. It is particularly designed to identify heterozygous bases in the
electropherogram traces. The algorithm also assigns calibrated quality values for
each consensus base to help you review data quality.
Analyzing Data with MicroSeq
®
ID Analysis Software
Note: MicroSeq
®
ID Analysis Software is specialized for bacterial and fungal
identification and is not suitable for generalized sequence analysis. Ask your Applied
Biosystems field application specialist for more information.
Overview and
Applications
MicroSeq ID Analysis Software is a tool for identification of bacteria and fungi. The
software analyzes data generated using a MicroSeq
®
chemistry kit and an Applied
Biosystems capillary-based genetic analyzer.
The MicroSeq ID Analysis Software:
1. Compares the DNA sequence from an informative region of the ribosomal RNA
to the sequences of known reference strains of bacteria or fungi stored in a
library.
2. Generates a final identification list of organisms that are the closest matches to
the unknown sequence from the ribosomal RNA.

189DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis
3. Reports the percent similarity that reflects how closely the unknown isolate
matches the library sequence
Software
Workflow
1. Create a project.
2. Analyze the project.
3. Evaluate the results.
4. If needed, edit the data in the analyzed project.
5. Export and print reports.
For More
Information
See Chapter 8 for information on troubleshooting sequencing data.
Refer to the Applied Biosystems MicroSeq
®
ID Analysis Software v2.0 Getting
Started Guide (PN 4364623) and the online help for instructions.
To obtain the latest software updates and patches, go to
www.appliedbiosystems.com, then click the link for Support.

190 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 7 Data Analysis

193DNA Sequencing by Capillary Electrophoresis Chemistry Guide
8
Troubleshooting 8
This chapter covers:
Troubleshooting Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .194
Troubleshooting Workflow . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .194
Table of Troubleshooting Symptoms. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .201
Troubleshooting Examples . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .203
Troubleshooting Index. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .255

194 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Troubleshooting Overview
This chapter provides information for troubleshooting automated DNA sequencing
results from capillary electrophoresis runs.
Assumptions
Troubleshooting suggestions listed in this chapter assume the following:
• The instrument completed the run(s) and data are visible in Data Collection
Software
• Sample files were extracted successfully
• The run folder was created and saved on the instrument computer
• The correct number of *.ab1 sample files were created within the run folder
• *.ab1 sample files can be opened and viewed in an Applied Biosystems analysis
software program, such as Sequence Scanner or Sequencing Analysis Software
If these conditions are not met, you may have an instrument or Data Collection
Software problem. You may need to repeat data extraction and/or data analysis. Refer
to your instrument user guide to continue troubleshooting.
Using Controls
To simplify troubleshooting, Applied Biosystems recommends that you run controls
with every run for multicapillary instruments or each set of runs on 310 instruments:
• DNA template control (pGEM
®
-3Zf(+) or M13mp18) (page 64) – Results
can help you determine whether failed reactions are caused by poor template
quality or sequencing reaction failure.
• Sequencing standards (page 129) – Results can help you distinguish between
chemistry problems and instrument problems.
Troubleshooting Workflow
When troubleshooting, follow this workflow to identify the problem. In general,
check for the errors that can be resolved most easily. The figures in this section show
Sequencing Analysis Software examples, however you can use Sequence Scanner
Software. For more information, see “Analyzing Data with Sequencing Analysis
Software” on page 170.
1. Review the electropherogram (page 195).
2. Review data analysis settings (page 195).
3. Review run and data analysis information (page 196).
4. Review experimental setup (page 199).
5. Note any patterns in the occurrence of the problem. For example, does the
problem occur in specific capillaries, specific regions of the plate, an entire run,
or multiple runs?
6. If you have not resolved your problem, identify the symptom in “Table of
Troubleshooting Symptoms” on page 201. Then, determine the cause and
perform the actions to resolve the problem.
7. If the problem persists, contact Applied Biosystems Technical Support.

195DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Reviewing the
Electrophero-
gram
1. Open the sample file in Sequencing Analysis Software and select the
Electropherogram tab for the analyzed view. The analyzed view is rescaled.
Then go to the raw view.
2. Review the current, voltage, temperature, and power throughout the
electrophoresis run to determine whether an electrical problem occurred during
the run. Large fluctuations in the values can result in poor quality data.
Note: Sequencing Analysis 5.X rescales the raw data to improve peak visibility
in the Electropherogram view. Peak height in the Electropherogram view should
not be used as the only indicator of data quality.
Figure 34 Example of electropherogram with high quality data
Reviewing Data
Analysis Settings
Review data analysis settings using Sequencing Analysis Software.
1. In the Sample Manager, verify that the appropriate basecaller, mobility file
(dyeset/primer), and matrix file (310 instruments only) are used:
2. A change in nomenclature occurred between software versions. If the analysis
file is in bold, italic text, verify that the analysis files are in the appropriate
location:
• Basecaller file – In the same folder as the Sequencing Analysis Software
(for example: X:\Applied Biosystems\SeqA5.X\AppSeqA\bin\Basecaller\
Params)
• Mobility file – In the Mobility folder (for example: X:\Applied
Biosystems\SeqA5.X\AppSeqA\bin\Basecaller\Mobility)
• Matrix file – In the Matrix folder (for analysis of 310 instrument data) (for
example: X:\Applied Biosystems\SeqA5.X\AppSeqA\bin\Basecaller\
Matrix)
See Appendix C in the Applied Biosystems DNA Sequencing Analysis Software
User Guide for more information on troubleshooting these files.
Verify that the mobility file (DyeSet/Primer) is
appropriate for the basecaller
Matrix files are required
for 310 instruments only
Bold, italic text indicates the file is
not in the appropriate location

196 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
3. In the analysis protocol and settings, verify the basecaller settings.
Reviewing Run
and Analysis
Information
Review run and analysis information using Sequencing Analysis Software.
1. Click Show next to the sample you want to display.
2. Select the Raw tab and review the raw, unprocessed fluorescence data for the
sample to assess the signal quality. Check for the following:
• Artifacts – Are there any artifacts, such as four-color spikes? For an
example of spikes, see page 226.
• Peak heights – Are peaks well-resolved, with reasonable heights
(Figure 35)? For examples of low or no signal, see pages 207 through 211;
for examples of top-heavy data, see pages 222 through 225.
• Data start points – Do any data start points deviate from others in the run?
For examples of start point deviation, see pages 213 and 214.
• Length of read – Was the expected length of read obtained? Does the
signal stop suddenly? For examples of sudden, premature drops in signal,
see pages 219 through 221.
• Baseline – Is there background noise for all the peaks? Zoom in
horizontally and vertically to verify the baseline noise.
Figure 35 Example of high quality raw data with tightly resolved peaks from the
3730 Genetic Analyzer

197DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
3. Select the Annotation tab and review the data collection and data analysis
settings and values for the sample file (Table 34).
Table 34 Annotation tab information
Setting Comments
Data Collection Settings
Note: Incorrect Data Collection settings can result in basecalling errors during data
analysis.
Instrument Model Make sure that the run parameters were appropriate for the
instrument model (for more information, see page 151).
Length to Detector Capillary length. If the incorrect length was set, peaks can begin
later than expected (for an example, see page 213).
Note: For 310 instruments, the length to detector value does not
affect data analysis.
Run Module Name If the incorrect run module was used, peaks can begin later than
expected (for an example, see page 213) or basecalling may be
affected.
Data Analysis Settings
Basecaller Name For more information about the basecallers, see page 144.
DyeSet/Primer or
Mobility File
If the incorrect mobility file was applied in the analysis, peaks are
not evenly spaced, especially peaks in the first 100 to 150 bases
(for an example, see page 229) and/or base assignments may be
incorrect.
Ave Signal Intensity Low or high values can produce low quality data (for examples,
see pages 231, 233, page 239, and page 242).
Generally acceptable values:
• 3730/3730xl instruments: 500 to 10,000 rfus
• 310 and 31XX instruments: 50 to 1000 rfus
Note: The values listed above are not specifications.
Signal:Noise Average relative fluorescent units (rfu) divided by the noise level for
each dye. High quality data normally yields a signal to noise ratio
>100, although accurate basecalling can be achieved with values
as low as 25.
Base Spacing Used A negative number indicates abnormal peak spacing values.
Basecalling may not be accurate for the sample.

198 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
4. Select the EPT tab and review the current, voltage, temperature, and power
throughout the electrophoresis run to determine whether a gross electrical
problem occurred during the run. Large fluctuations in the values can result in
poor quality data.
Figure 8-36 Example of EPT tab information for high quality data
Run voltage (volts/100)
Run current (microAmps)
Run temperature ( °C)
Power (mWatts*10)
Prerun
Run conditions
Injection

199DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Reviewing
Experimental
Setup
1. Confirm that you used the optimal quality and quantity of DNA using Table 35.
Table 35 Reviewing DNA quality and quantity checklist
✓ Recommendation Comments
Run an agarose gel to detect any
contaminating DNA or RNA.
Purified DNA runs as a single band on an
agarose gel.
Note: Uncut plasmid DNA can run as
three bands: supercoiled, nicked, and
linear.
Note: RNA contamination up to 1 µg
can be tolerated in the sequencing
reaction, but it affects DNA quantitation
greatly.
Measure the A
260
/A
280
ratio of your
samples.
For pure preparations of DNA (in TE), the
A
260
/A
280
ratio is 1.8. For pure
preparations of RNA (in TE), the ratio is
2.0. Very clean samples in pure water can
give a ratio of 1.5 to 1.6. (Sambrook et al.,
1989
Smaller ratios may indicate the presence
of protein or organic contaminants.
Ratios less than 1.8 may still produce
high quality results.
Quantitate the DNA template using the
absorbance at 260 nm (A
260
).
Quantitation by agarose gel
electrophoresis may not be accurate
because ethidium bromide incorporation
is not consistent and the method of
comparing the standard and sample
brightness is subjective.
Dilute or concentrate the DNA as needed
to obtain an A
260
reading between 0.05
and 1.00.
A
260
values below 0.05 or above 1.00 are
not accurate because Beer’s law
generally applies only within a certain
concentration range. Outside of this
concentration range, the relationship
between absorbance and concentration
is nonlinear.
Use the amount of DNA template in
Table 8, “Recommended DNA template
quantities for cycle sequencing,” on
page 63.
Calculate the template concentration
using the formulas on page 45.
Too little template can result in no or low
signal.
Too much template can result in top
heavy data (page 222 through 225).
Use the primer concentrations
recommended in Chapter 4: 3.2 pmol in a
20-μL reaction (dye terminator chemistry).
Calculate the primer concentrations using
the formula on page 39.
Too little primer can result in no or low
signal (pages 207 through pages 211).
Too much primer can lead to
overamplification of the 5′ end of the
template, resulting in top heavy data
(page 222 and 224).

200 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
2. Confirm that the primer design and quality are optimal using Table 36.
Table 36 Reviewing primer design checklist
✓ Recommendation Comments
Ensure that the primer has T
m
>45 °C. If the T
m
is too low, it may result in poor
priming and low or no signal (pages 207
through pages 211).
Ensure that primers are at least 18 bases
long.
Primers that are too short may have T
m
s
that are too low.
Ensure that there are no known
secondary hybridization sites on the
target DNA.
Secondary hybridization sites on the
target DNA can result in double peaks
throughout the sequence (page 237).
Choose primers that do not have runs of
identical nucleotides, especially 4 or more
Gs.
Runs of identical nucleotides in primers
can cause n+1 or n-1 effects (page 244).
Also, these primers may be more difficult
to synthesize.
Choose primers with G-C content in the
range of 30 to 80%, preferably 50 to
55%.
If the G-C content is too low, the T
m
may
be too low. If so, increase the primer
length beyond 18 bases to obtain a
T
m
>45 °C.
Design primers to minimize the potential
for secondary structure and/or
hybridization (see page 38).
Primer-dimer formation from hybridization
can result in mixed sequence at the
beginning of the sequence (page 240).
Secondary structure in the primer,
particularly at the 3′ end can result in poor
priming and low or no signal (pages 207
through pages 211).
Purify primers by HPLC to reduce the
quantity of n-1 primers.
Primers containing contaminants or
synthesized primers of the wrong length
can cause problems in sequencing
reactions, such as failed reactions, noisy
data, or poor sequencing results. If the
primer is a short oligo that contains
n-1 primers, HPLC cannot always remove
the n-1 contaminants.

201DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Table of Troubleshooting Symptoms
The table below lists troubleshooting symptoms and a page reference for an example
of the symptom and possible causes and actions to take to resolve the problem. If
there are two or more possible causes for the symptom, the causes are grouped and
listed in the following order: data analysis issues, electrophoresis issues, then
sequencing reaction issues.
Table 37 Table of troubleshooting symptoms
Symptom
Example
on Page
Sample Manager Errors
Spacing value is red in Sequence Analysis or Sequence Scanner Software 203
Incorrect Basecalling
Mixed base not called correctly 204
Too many mixed bases called 205
Irregular Signal
No signal or low signal:
• No signal
• Low signal
• Low signal throughout
207
209
211
Signal starts later than expected:
• Signal starts later than expected: no resolution loss
• Signal starts later than expected: with resolution loss
213
214
Irregular baseline:
• Negative baseline: one color
• Negative baseline: all four bases
• Waterfall baseline
216
217
218
Sudden drop in signal:
• Sudden drop in signal: corresponds to basecalling stop when sequencing short template
• Sudden drop in signal: early sudden drop with sequence termination
• Sudden drop in signal: sudden drop with continued basecalling
219
220
221
Top-heavy data:
• Top-heavy data: gradual loss of signal
• Top-heavy data: ski slope profile
• Top-heavy data: preferential amplification of short sequence
• Top-heavy data: split peaks with excessive signal
222
223
224
225

202 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Abnormal Peak Shapes
Spikes:
• Four-color spikes
• One-color spikes
• Large spike at the end of the run
226
227
228
Improperly spaced peaks, especially peaks in the first 100 to 150 bases 229
Large peaks (blobs) in the first 120 bases 230
Irregular C peaks using BigDye® Terminators v3.1 231
Irregular G peaks using BigDye® Terminators v1.1 and 3.1 233
Shoulders on all peaks 234
Peak compressions 235
Broad peaks for bisulfite-converted sequences 236
Double peaks:
• Double peaks: peaks under peaks throughout
• Double peaks: with high average signal intensity values
• Double peaks: at the beginning of the sequence
• Double peaks: at the beginning of the sequence (bisulfite conversion)
• Double peaks: specific peaks under specific bases
• Double peaks: specific peaks under specific bases
• Double peaks: peaks under peaks throughout (bisulfite conversion)
• Double peaks: after a homopolymer or repeated sequence
• Double peaks: after a homopolymer or repeated sequence (bisulfite sequencing)
• Double peaks: double sequence after clean sequence
237
239
240
241
242
243
244
246
247
248
Low Resolution
Resolution loss: at beginning of run 249
Resolution loss: in the middle of the run 250
Resolution loss: gradual early loss 251
SeqScape Software Symptoms
High quality sequence in unassembled category in SeqScape Software 253
Table 37 Table of troubleshooting symptoms (continued)
Symptom
Example
on Page

203DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Troubleshooting Examples
Spacing value is red in Sequence Analysis or Sequence Scanner Software
Red spacing value in
Sample Manager
Possible Cause(s) Recommended Action
Data analysis issue: The red color indicates that the
basecaller applied a default value for spacing. The
basecaller determined that the sample cannot be
analyzed because the spacing estimation algorithm failed.
This error may occur if the data has been collected using
modified run modules or if data are poor.
Verify that analysis settings are appropriate for the run
setup.
Manually set a spacing value and reanalyze the data. To
estimate a spacing value:
1. Refer to the raw data after 1000 scan points.
2. Measure the distance between the crests of two
adjacent peaks with the same color.
For more information, see the appropriate Sequencing
Analysis Software user guide.

204 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Mixed base not called correctly
Ns or low QVs for pure bases
are assigned instead of
mixed bases (analysis using
the KB basecaller only)
Electropherogram
Possible Cause(s) Recommended Action
Data analysis issue: The quality threshold setting and
the mixed bases settings are not correctly defined in the
analysis protocol.
1. Review the quality threshold setting (page 149) and the
mixed bases settings (page 149) in the analysis
protocol that you used for the analysis.
2. Correct the settings if necessary, then reanalyze the
data.
Note: Significant improvements in mixed basecalling
have been made with later versions of Sequencing
Analysis Software and the KB basecaller. Please check
the Applied Biosystems web site for the latest updates.

205DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Too many mixed bases called
Too many mixed bases are
called (analysis using the KB
basecaller only)
Electropherogram
Possible Cause(s) Recommended Action
Data analysis issues:
2nd highest peak threshold for mixed base identification
is set too low. The recommended range is 15 to 25%.
Review the Mixed Bases settings in the analysis protocol
that you used for the analysis (page 149). Change the
settings if necessary, then reanalyze.
Electrophoresis issues (likely in multiple lanes and/or runs):
Carryover from contaminated septa. Replace septas and change buffer, water, and waste.
Electrical noise. Check the uninterruptible power supply (UPS).
Contaminated water or buffer because of dirty containers,
microbial growth, or use of tap water for cleaning.
Clean all reservoirs, upper and lower polymer block, and
septa with deionized water.
Poor or incorrect spectral calibration (spectral pullup). Perform the spectral calibration again.
Shifted spatial calibration. Perform the spatial calibration again.
Poor CCD alignment. Contact Applied Biosystems to arrange a service
engineer visit.
Secondary primer site in the template was sequenced. Design a new sequencing primer (page 38).
Secondary amplification product in the PCR product used
as a sequencing template.
Use gel purification to isolate the desired product. For
more information, see “Purifying PCR Products for
Sequencing” on page 41.
Design new PCR primers or optimize amplification
parameters to obtain a single product. For more
information, see “Preparing PCR DNA Templates” on
page 37.
PCR primers were not completely removed from the PCR
product used as a sequencing template.
Remove PCR primers completely before using PCR
products as sequencing templates. For more information,
see “Purifying PCR Products for Sequencing” on
page 41.
Mixed templates. Review the DNA quality.

206 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Pull-up caused by overloading the capillaries with too
much product.
Review DNA quantity.
Use standard run modules
Click the Annotation tab and examine the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Load less labeled sample by performing one of the
following:
• Remove some of the sample and replace with Hi-Di
™
Formamide
• Inject sample for less time
• Resequence the samples, using less template in the
sequencing reaction, especially if you use the BigDye
®
XTerminator
™
Purification Kit (see Tab le 8,
“Recommended DNA template quantities for cycle
sequencing,” on page 63).
Stutter during either PCR amplification and/or cycle
sequencing. Stutter is most common in any
homopolymeric region greater than 2 bases. It can also
be seen with simple repeated DNA sequences. The
results are worse when the stutter occurs during PCR
amplification.
It is thought that stutter occurs when a partially extended
primer and template dissociate, then reanneal improperly
before extension continues. Partially extended primers
and templates commonly dissociate during the reaction,
but if they reanneal with complete fidelity, the reaction
produces only one product. Improper annealing results in
one or more products that are represented in the
sequencing results.
If stutter occurs during PCR amplification, little can be
done to correct the problem, except using anchored
sequencing primers.
If stutter occurs during cycle sequencing:
• Try using dRhodamine terminators. They have been
shown to be less prone to produce stutters,
specifically with poly-T regions.
• Some customers have found that they can get past
poly(A) regions using a mixture of oligo dT
18
primers
with either a C, A, or G as the 3′ terminal dinucleotide
or 2-base anchors.
Possible Cause(s) Recommended Action
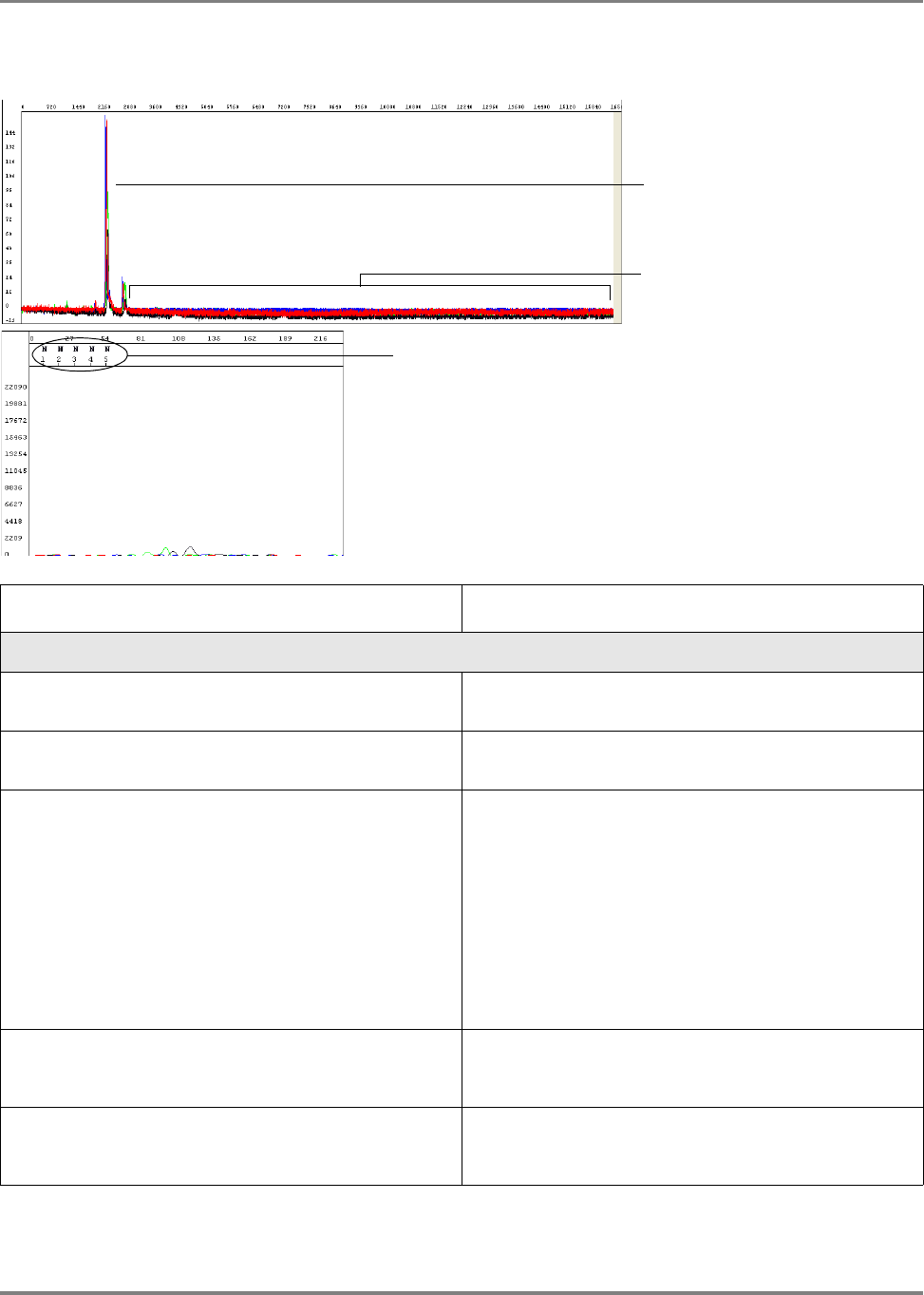
207DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
No signal
Flat signal profile
Raw Data
KB basecaller generated 5 Ns to indicate a
possible failed sequencing sample
Electropherogram
Unincorporated dyes
Possible Cause(s) Recommended Action
Loss of labeled product during purification of extension
products.
See Chapter 5 for suggestions on retaining labeled
product during purification.
Thermal cycler malfunction. Determine with the manufacturer how to test your thermal
cycler for proper performance.
One of the components of the sequencing reaction
(template, primer, or Ready Reaction Mix) was either
omitted, was the wrong material, or was of poor quality.
Review the entire experiment carefully.
1. Check the quantitation and quality of the sequencing
reaction components.
2. For each component, replace the component, perform
a sequencing run, then evaluate the results until you
have identified the problem or replaced all of the
reaction components.
3. Run a DNA template control to determine whether the
sequencing reaction failed or the template quality is
low (page 64).
Insufficient template added to sequencing reactions,
leading to too few sequencing products generated during
PCR.
Check DNA quantitation and quality (page 44 and 45).
Template contains sequencing inhibitors such as phenol
(page 44).
Follow recommended procedures to prepare templates.
Check DNA quality (page 44). If necessary, clean up dirty
templates.

208 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
No enzyme activity because Ready Reaction Mix was
stored improperly or it separated upon storage.
Check the color of the Ready Reaction Mix. If the color is
not uniform, the Ready Reaction Mix separated upon
storage. Mix the Ready Reaction Mix gently before using
it.
Run a DNA template control to test enzyme function
(page 64).
Weak priming because of poor primer design. Review primer design (page 38). Make new primers, then
repeat the sequencing experiment.
Possible Cause(s) Recommended Action

209DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Low signal
Possible Cause(s) Recommended Action
Electrophoresis issues:
One or more broken or blocked capillaries. Visually check the capillaries. If any are broken or
blocked, replace the entire array. If subsequent runs show
failure in the same capillary, replace the entire array.
Check the results using the long read sequencing
standard.
Optical path is obstructed (3100/3100-Avant instruments
only).
Check the laser power, using the EPT in Data Collection
Software. Perform the spatial calibration again.
Check whether you can hear the shutter clicking during
data collection. If you cannot hear it click, contact Applied
Biosystems for a service engineer visit.
If all capillaries show no signal or low signal, contact
Applied Biosystems for a service engineer visit.
Electropherogram shows
Ns (with ABI or KB
basecaller) or low quality
bases (with KB basecaller)
Electropherogram
Raw Data

210 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Sample evaporated because water was used as the
injection solution.
Use Hi-Di
™
Formamide to resuspend your samples (see
page 122).
For future experiments, consider using the BigDye
®
XTerminator
™
Purification Kit to purify samples (see
page 88).
Use a heat sealer to seal the plates (3730/3730xl
instruments only).
Add more resuspension solution to the samples before
loading them.
Sample volume is too low. Resuspend samples using sufficient volumes (at least
10 μL) (see page 122).
Autosampler alignment is off and the tips did not enter the
sample.
1. Verify the correct run module was used.
2. If you are using samples purified with BigDye
®
XTerminator
™
Purification Kit and your autosampler
was recently calibrated, run the BDX Update utility.
Select StartAll ProgramsApplied
BiosystemsBDX Updater. (The utility is installed
with the BigDye XTerminator run modules.)
3. Contact Applied Biosystems to arrange a service
engineer visit.
Slightly unstable current and voltage during
electrophoresis.
Check the current and voltage.
Buffer is old. Replace the buffer according to the procedures in your
instrument user guide.
Too much template or sample temporarily clogging the
capillary.
Reinject the sample.
Injection failed. • Verify correct run module was used.
• Verify correct volume in well.
• Verify capillaries are not broken or blocked.
Possible Cause(s) Recommended Action

211DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Low signal throughout
Low signal throughout
the entire sequence
Electropherogram
Raw Data
Annotation tab shows low average signal intensity values
for data from 3730 instrument
Possible Cause(s) Recommended Action
Sequencing reaction failed. Check the control template and primer.
Partial loss of labeled products during purification of
extension products.
See Chapter 5 for suggestions on retaining labeled
product during purification.
Sample contains salts from insufficient purification of
templates, PCR products, or sequencing reactions with
ethanol precipitation. Salts in the sample interfere with
proper electrokinetic injection.
Review DNA quality, PCR purification, and sequencing
reaction purification steps.
The amount of Ready Reaction Mix in the reactions was
insufficient, usually because the sequencing chemistry
was diluted.
Follow recommended procedures to prepare sequencing
reactions with Ready Reaction Mixes. See page 66 for
recommended procedures. Applied Biosystems does not
support diluted reactions or guarantee the performance of
diluted BigDye chemistry.
Not enough primer or template in the cycle sequencing
reaction.
Review DNA quantity (page 199). Use the amounts
recommended on page 63. Run a DNA template control
to check sequencing reaction quality (page 64).

212 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Poor template quality. Follow recommended procedures to prepare templates.
Check DNA quality (page 44). If necessary, clean up dirty
templates. Run a DNA template control to check
sequencing reaction quality (page 64).
Failure caused by difficult template sequence. Use Table 7 on page 56 to select a chemistry kit for
certain difficult templates.
Possible Cause(s) Recommended Action

213DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Signal starts later than expected: no resolution loss
Data starts later than expected
Possible Cause(s) Recommended Action
Electrophoresis issues:
Incorrect capillary length (Length to Detector) or run
module was selected.
1. Review run information in the Annotation tab using
Sequencing Analysis Software (see page 197):
– Length to Detector
–Run module
2. If an incorrect selection was made, run the samples
again using the correct settings.
Variation in lab temperature leads to faster or slower runs. Stabilize the lab temperature.
Sample heated during vortexing step of BigDye
®
XTerminator
™
purification.
1. Repeat the sequencing reactions.
2. Perform BigDye XTerminator purification using
recommended vortexer and plate adapter.
3. Run the samples again.
Too much template used. Run the samples again, using less template.

214 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Signal starts later than expected: with resolution loss
Data starts later than expected
Raw Data
Loss of resolution
Electropherogram
Peaks are broad in the region
of resolution loss marked
above
Possible Cause(s) Recommended Action
Electrophoresis issues:
Capillaries overloaded with sequencing product, possibly
unlabeled DNA or RNA.
Click the Annotation tab and examine the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Re-inject the samples using decreased injection time
and/or lower voltage.
Load less labeled sample by using less template in the
sequencing reaction (see Table 8, “Recommended DNA
template quantities for cycle sequencing,” on page 63).
Temperature in room and/or oven fluctuating. Review the EPT tab using Sequencing Analysis Software
(see page 198). If the oven temperature is fluctuating, the
oven may be leaking because of a poor seal. Contact
Applied Biosystems to arrange a service engineer visit.
Contaminant migrated through the capillary during
electrophoresis.
Run the sample again.
Capillary not filling. Check the pin valve in the polymer block, amount of
polymer in the bottle, leaks in the check valves, and
polymer pump function. Contact Applied Biosystems to
arrange a service engineer visit.
Temperature in the array heater fluctuating more than
±0.5 °C (3730/3730xl and 3130/3130xl instruments and
POP-7 only).
Using Data Collection Software, check the array heater
temperature. If it fluctuates more than ±0.5 °C, contact
Applied Biosystems to arrange a service engineer visit.
215DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Water in polymer system caused by insufficient flushing
after water wash maintenance.
Flush the polymer, using the wizard if possible.
Extension products purified using bead-based kits were
injected without removing the magnetic beads. The beads
may interfere with the extension products during injection
and cause overloading or other injection anomalies.
Remove magnetic beads before loading the sample.
Variables that affect current set incorrectly. • Replace buffer in system with fresh 1✕ running buffer.
• Inspect system for leaks (wet or dry polymer around
fitting indicates a leak) and tighten fittings as needed.
• Look for discoloration in the block channels or tubing.
If present, perform a water wash on the system using
the wizard in Data Collection Software.
Possible Cause(s) Recommended Action

216 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Negative baseline: one color
Baseline fluorescence for
one color is below 0 rfus
Raw Data
Software corrects the
baseline fluorescence during
data analysis
Electropherogram
Possible Cause(s) Recommended Action
You are using an early version of Sequencing Analysis
Software. This error, found in versions earlier that v5.2,
was corrected in Basecaller updater v2.0.
Upgrade Sequencing Analysis Software v5.2 or later.
To obtain the latest software updates and patches, go to
www.appliedbiosystems.com, then click the link for
Support.

217DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Negative baseline: all four bases
Raw Data
Baseline fluorescence for all
four colors is below 0 rfus
Possible Cause(s) Recommended Action
Electrophoresis issue: Excessive fluorescent
contamination in the detection area that bleaches out
over the duration of the run (3730/3730xl instruments
only).
Use manual control to turn on the laser before starting the
run to negate the effects of excessive fluorescent
contaminant. Contact Applied Biosystems technical
support or a field applications specialist.
Perform a water wash on all components of the system
using the wizard in Data Collection Software, then replace
the capillary array.

218 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Waterfall baseline
Signal dropout at point of
waterfall
Raised baseline in all colors
Baseline for all colors falls in a
“waterfall” pattern
Raw Data
Possible Cause(s) Recommended Action
Residue from cleansers used on glassware.
Note: Primarily observed in syringe-based instruments.
Rinse all components with deionized water.
Check gasket on syringe(s), replace if necessary.
Replace syringe(s).

219DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Sudden drop in signal: corresponds to basecalling stop when sequencing
short template
Sudden drop in signal
Raw Data
Basecalling stops when the
signal drops
Electropherogram
Possible Cause(s) Recommended Action
Data analysis issue: The drop in signal identifies a PCR
stop point and the basecaller stops calling bases beyond
this point. With the ABI basecaller, you observe Ns
beyond the PCR stop. With the KB basecaller, the
analyzed trace is displayed until the last basecall.
Select the At PCR Stop check box in the analysis
protocol using Sequencing Analysis Software (see
page 148).

220 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Sudden drop in signal: early sudden drop with sequence termination
Early sudden drop in signal
Raw Data
Early sudden termination
of sequence
Electropherogram
Possible Cause(s) Recommended Action
DNA polymerase enzyme stopped at a region of the
template that was difficult to sequence.
• Depending on sequence contexts, you can try
sequencing some template with dGTP kits (if the
problem sequence is G-rich), dRhodamine kits, or
BigDye primer kits.
• If termination of sequencing was caused by hairpins or
secondary structure, redesign primers around the
problem region. Some customers report that certain
additives can help, but Applied Biosystems cannot
recommend any specific protocols.
Not enough Ready Reaction Mix was used in the
sequencing reaction.
Follow recommended procedures to prepare sequencing
reactions with Ready Reaction Mixes. See page 66 for
recommended procedures. Applied Biosystems does not
support diluted reactions or guarantee the performance of
diluted BigDye chemistry.

221DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Sudden drop in signal: sudden drop with continued basecalling
Sudden drop in signal
Raw Data
Basecalling continues
beyond the drop in signal
Electropherogram
Possible Cause(s) Recommended Action
DNA polymerase had difficulty processing through a
particular sequence context.
Depending on sequence contexts, you can try
sequencing some template with dGTP kits, dRhodamine
kits, or BigDye
®
primer kits.
Not enough Ready Reaction Mix was used in the
sequencing reaction.
Follow recommended procedures to prepare sequencing
reactions with Ready Reaction Mixes. See page 66 for
recommended procedures. Applied Biosystems does not
support diluted reactions or guarantee the performance of
diluted BigDye chemistry.
If the problem persists, try sequencing using the dGTP
kits.

222 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Top-heavy data: gradual loss of signal
Early peaks are taller
Peak heights fall to zero
before the end of the
sequence
Peak height decreases
Large number of small molecular weight peaks in the sequencing reaction
Raw Data
Possible Cause(s) Recommended Action
Improper cycling conditions for extension. The extension
time is too short or the extension temperature is too high.
Increase the extension time or decrease the extension
temperature.
Improper ratio of primer to template in the sequencing
reaction.
Set up a matrix of reactions with varying ratios of
primer:template to determine which ratio produces the
best peak profile.
Sequencing template contains a contaminant that inhibits
DNA polymerase activity.
Review how templates are prepared. Try a different
method or clean up dirty templates (page 44).
Not enough Ready Reaction Mix was used in the
sequencing reaction.
Follow recommended procedures to prepare sequencing
reactions with Ready Reaction Mixes. See page 66 for
recommended procedures. Applied Biosystems does not
support diluted reactions or guarantee the performance of
diluted BigDye chemistry.
Template or extension products are degraded. With
degraded extension products, the data are noisy, with a
higher baseline at the start of peaks.
Review how templates are prepared and stored. Try a
different method (Chapter 3) and store at − 20 °C.

223DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Top-heavy data: ski slope profile
Peak profile is similar to a
ski slope: peak heights
decrease as the fragment
lengths increase
Raw Data
Possible Cause(s) Recommended Action
Not enough or too much template was used in the
sequencing reaction.
Review the DNA quantity (page 199).
Not enough or too much primer was used in the
sequencing reaction.
Not enough Ready Reaction Mix was used in the
sequencing reaction.
Follow recommended procedures to prepare sequencing
reactions with Ready Reaction Mixes. See page 66 for
recommended procedures. Applied Biosystems does not
support diluted reactions or guarantee the performance of
diluted BigDye chemistry.
Template is degraded. Review how templates are prepared and stored. Try a
different method (Chapter 3) and store at − 20 °C.

224 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Top-heavy data: preferential amplification of short sequence
Short 5′ sequence, instead
of the entire template, is
amplified preferentially, (see
also page 240)
Raw Data
Possible Cause(s) Recommended Action
during the PCR reaction.
Redesign the PCR primers to eliminate the sequences
that allow primer-dimer formation.
Use a “hot start” PCR enzyme to inhibit primer-dimer
formation.

225DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Top-heavy data: split peaks with excessive signal
Peaks with excessive
signal
Split peaks and
pullup at the
beginning of the
sequence in the
region of peaks with
excessive signal
(circled above)
Electropherogram
Raw Data
Possible Cause(s) Recommended Action
Signal is too high because too much template was used
in the sequencing reaction and too much sequencing
product was created.
You do not have to repeat the reaction.
Click the Annotation tab and check the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Especially if your samples were purified using the
BigDye
®
XTerminator
™
Purification Kit, load less labeled
sample by performing one of the following:
• Remove some of the sample and replace with Hi-Di
™
Formamide
• Inject sample for less time
• Resequence the samples, using less template in the
sequencing reaction, especially if you use the BigDye
®
XTerminator
™
Purification Kit (see Tab le 8,
“Recommended DNA template quantities for cycle
sequencing,” on page 63).
For future reactions, reduce the amount of template in the
sequencing reaction.
Injection height incorrect due to incorrect run module. Use correct run module, especially for samples purified
with the BigDye XTerminator Purification Kit (see
“Selecting a Run Module” on page 137).

226 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Four-color spikes
Four-color spikes
Raw Data
Four-color spikes
Electropherogram
Electropherogram (zoomed in)
Zoomed in electropherogram
shows spikes contain all four
colors
Possible Cause(s) Recommended Action
Electrophoresis issues:
Dust, bubbles, or crystals in polymer passed through the
path of the laser beam.
1. Eliminate large amounts of dust in the environment.
2. Inspect the upper gel block for bubbles. If present,
flush all bubbles out of the system and out of the array
manually.
3. Check the polymer bottle for crystals. If present, warm
the polymer gently to 30 °C with gentle mixing, then
refill the syringes and array with the polymer.
4. Replace polymer if the condition persists.
Polymer is expired or was stored at room temperature for
more than 7 days.
Replace the polymer.

227DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
One-color spikes
One-color positive spikes
One-color negative spikes
Raw Data
Possible Cause(s) Recommended Action
Electrical noise or power fluctuations. Verity the power source, use uninterruptible power
supply.
Polymer temperature is too high. • Verify the shipping temperature of the polymer.
• Verify lab temperature is below 26 °C.
Well volume is too low. • Verify volume is ≥10μL for 96-well plates and ≥15μL for
384-well plates.
• If using septa, verify septa are fresh to minimize
evaporation.

228 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Large spike at the end of the run
Large spike at end of run
Raw Data
Possible Cause(s) Recommended Action
The large spike at the end of the run, called a reptation
peak, occurs with almost all electrophoretic separations
of DNA on capillary instruments. With typical run
conditions, data collection stops well before the spike
occurs. There is no useful sequencing information in the
spike or just before the spike. Because some run modules
are designed for the longest possible read lengths, data
collection stops just before the spike occurs. Normal run
variation within a lab may result in the spike appearing in
some electropherograms.
None needed. Shorten the data collection time a few
minutes to remove a persistent spike from your data.

229DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Improperly spaced peaks, especially peaks in the first 100 to 150 bases
Peaks are improperly
spaced, especially peaks in
the first 100 to 150 bases
Electropherogram
Possible Cause(s) Recommended Action
Data analysis issue: Wrong mobility file applied to the
sequence data.
Reanalyze the data using the correct mobility file to
observe proper spacing of all peaks.
For more information about mobility files, see page 145.

230 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Large peaks (blobs) in the first 120 bases
Blobs in the first 120 bases
Precise location of the blobs
varies according to the dye
used and the specific
configuration
Electropherogram
Possible Cause(s) Recommended Action
Incomplete removal of dye-labeled terminators after the
cycle sequencing reaction.
Review the methods described in Chapter 5, “Purification
of Extension Products.” If you are using a third-party
product for purifying extension products, contact the
manufacturer for troubleshooting help.
For future experiments, consider using the BigDye
®
XTerminator
™
Purification Kit to purify samples (see
page 88).
Poor incorporation of terminators, leaving excess
unincorporated terminators.
Review the entire experiment carefully.
• Check the quantitation (page 45).
• Check the quality of the sequencing components.
Replace each component, one at a time.
• Run a DNA template control to determine whether the
sequencing reaction failed or the template quality is
low (page 64).
• Check expiration dates on all reagents and replace any
that have expired.
If using BigDye XTerminator Purification Kit, insufficient
mixing during vortexing step.
• Verify plate is firmly attached to vortexer.
• Follow protocol for vortexing.
If using BigDye XTerminator Purification Kit, incorrect ratio
of BigDye XTerminator reagents.
• Vortex the XTerminator Solution bulk container at
maximum speed for at least 10 seconds before
dispensing.
• Use wide-bore pipette tips to dispense the
XTerminator Solution.

231DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Irregular C peaks using BigDye
®
Terminators v3.1
C peaks have shoulders
Electropherogram
C peaks are small and
rough
Electropherogram
No C peaks
Electropherogram
Ave Signal Intensity for C is lower than for G, A, or T
Possible Cause(s) Recommended Action
The dye labels attached to the ddC terminators are
degraded. Initial degradation results in shoulders on all C
peaks. With further degradation, the C peaks appear very
small or rough or disappear completely.
Protect the fluorescently labeled DNA from light, heat,
acidic conditions, and oxygen (see “Storing Sequencing
Reactions” on page 121).
If no C peaks are visible, repeat the sequencing reactions
with fresh reagents.
The Hi-Di
™
Formamide is degraded. Resuspend the samples using a newer lot of Hi-Di
Formamide.
Sequencing reactions were exposed to light, heat, acidic
conditions, and/or oxygen before they were loaded onto
the instrument.
Use tube septa or a heat seal to prevent exposure to air
and evaporation of samples, especially if you place the
samples in the autosampler more than 6 hours before
starting electrophoresis.
Verify that the primer and template pHs are not acidic.

232 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Electrophoresis issue:
The buffer heater is powered on (3730/3730xl instruments
only).
Verify that the buffer heater is not powered on.
Severe arcing events can mask the C signal. • Perform several water washes using the wizard in Data
Collection Software.
• Disassemble the system and clean out all components
with warm water (<42 °C).
Possible Cause(s) Recommended Action

233DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Irregular G peaks using BigDye
®
Terminators v1.1 and 3.1
Irregular G peaks
Electropherogram
Possible Cause(s) Recommended Action
Electrophoresis issue: The buffer heater is powered on
(3730/3730xl instruments only).
If you are using the 3730 instrument, confirm that the
buffer heater is not powered on.
The Hi-Di
™
Formamide is degraded. Resuspend the samples using a newer lot of Hi-Di
Formamide.
Sequencing reactions were exposed to light, heat, acidic
conditions, and/or oxygen before they were loaded onto
the instrument.
Use tube septa or a heat seal to prevent exposure to air
and evaporation of samples, especially if you place the
samples in the autosampler more than 6 hours before
starting electrophoresis.
Verify that the primer pH and the template pH are not
acidic.
The dye labels attached to the ddG terminators are
degraded. As shown in the figure above, the pattern for
degradation of dye labels on ddG terminators is different
than for ddC terminators. The G peak patterns are very
irregular, and the complexity increases as degradation
progresses.
This problem can occur with BigDye Terminators v1.1 and
less frequently with BigDye Terminators v3.1.
Protect the fluorescently labeled DNA from light, heat,
acidic conditions, and oxygen (see “Storing Sequencing
Reactions” on page 121).
Water used as Injection solution.
Note: Resuspending samples in water leads to
breakdown of C and/or G-labeled fragments.
Degradation of the dye labels attached to the ddG
terminators is less likely to occur in Hi-Di Formamide or
0.1 mM EDTA.

234 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Shoulders on all peaks
All peaks show shoulders
Electropherogram
Possible Cause(s) Recommended Action
Electrophoresis issues:
Capillary array needs to be replaced. Replace the capillary array.
Overloaded sample. Shorten the injection time.
Amplify less DNA.
Contamination of the sequencing primer with n+1 or n-1
sequencing primer.
Use the primers with a different template. If the problem
persists, resynthesize the primers before repeating the
experiment.
Stutter during either PCR amplification and/or cycle
sequencing. Stutter is most common in any
homopolymeric region greater than 2 bases. It can also
be seen with simple repeated DNA sequences. The
results are worse when the stutter occurs during PCR
amplification.
It is thought that stutter occurs when a partially extended
primer and template dissociate, then reanneal improperly
before extension continues. Partially extended primers
and templates commonly dissociate during the reaction,
but if they reanneal with complete fidelity, the reaction
produces only one product. Improper annealing results in
one or more products that are represented in the
sequencing results.
If stutter occurs during PCR amplification, little can be
done to correct the problem, except using anchored
sequencing primers.
If stutter occurs during cycle sequencing:
• Try using dRhodamine terminators. They have been
shown to be less prone to produce stutters,
specifically with poly-T regions.
• Some customers have found that they can get past
poly(A) regions using a mixture of oligo dT
18
primers
with either a C, A, or G as the 3′ terminal dinucleotide
or 2-base anchors.
Blending Ready Reaction Mixes from dGTP BigDye
terminator kits with BigDye terminator vx.1 kits.
Do not use blended Ready Reaction Mixes of dGTP
BigDye terminator kits and BigDye Terminator vx.1 kits.

235DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Peak compressions
Peak compressions
Electropherogram
Possible Cause(s) Recommended Action
Sequencing reaction issue: Observed when sequencing
GC-rich regions using dGTP sequencing chemistry.
Thought to result from incomplete denaturation of the
synthesized DNA.
No corrective action is known at this time. Some
customers report that using BigDye primers corrects this
problem.

236 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Broad peaks for bisulfite-converted sequences
Broad peaks in
sequence with partial
bisulfite conversion
Same sequence
before bisulfite
conversion
Possible Cause(s) Recommended Action
uneven because the sample contains both Cs (from
methylated samples) and no Cs (from unmethlyated
samples).
• Repeat bisulfite conversion
• Ensure amplicon is 250 to 400 bp for cloning and 100
to 250 bp for direct sequencing.
• Include and extra incubation at the end of thermal
cycling run for non-templated A addition.

237DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: peaks under peaks throughout
Peaks under peaks at every
position
Peaks are uniform
Electropherogram
Possible Cause(s) Recommended Action
Electrophoresis issues:
Carryover from contaminated septa. Replace septas, then change buffer, water, and waste.
Electrical noise. Check the uninterruptible power supply (UPS).
Dirty containers and/or tap water were used to clean
instrument components, resulting in contaminated water
or buffer.
Clean the containers to be used for cleaning instrument
components, then rinse the containers thoroughly with
deionized water.
It is preferable to use deionized water to clean the
instrument components.
Shifted spatial calibration. Redo the spatial calibration.
Poor CCD alignment. Contact Applied Biosystems to arrange a service
engineer visit.
Poor or incorrect spectral calibration (spectral pull-up). Redo the spectral calibration.
Secondary primer site in the template was sequenced. Design a new sequencing primer (page 38).
Secondary amplification product in the PCR product used
as a sequencing template.
Use gel purification to isolate the desired product or
design new PCR primers to obtain a single product. For
more information, see “Preparing PCR DNA Templates”
on page 37.
PCR primers were not completely removed from the PCR
product used as a sequencing template.
Remove PCR primers completely before using PCR
products as sequencing templates. For more information,
see “Preparing PCR DNA Templates” on page 37.
Mixed or contaminated templates or primers. Review the DNA quality.

238 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Stutter during either PCR amplification and/or cycle
sequencing. Stutter is most common in any
homopolymeric region greater than 2 bases. It can also
be seen with simple repeated DNA sequences. The
results are worse when the stutter occurs during PCR
amplification.
It is thought that stutter occurs when a partially extended
primer and template dissociate, then reanneal improperly
before extension continues. Partially extended primers
and templates commonly dissociate during the reaction,
but if they reanneal with complete fidelity, the reaction
produces only one product. Improper annealing results in
one or more products that are represented in the
sequencing results.
If stutter occurs during PCR amplification, little can be
done to correct the problem, except using anchored
sequencing primers.
If stutter occurs during cycle sequencing:
• Try using dRhodamine terminators. They have been
shown to be less prone to produce stutters,
specifically with poly-T regions.
• Some customers have found that they can get past
poly(A) regions using a mixture of oligo dT
18
primers
with either a C, A, or G as the 3′ terminal dinucleotide
or 2-base anchors.
Very strong or offscale data Reduce the signal:
• Adjust the injection time and/or lower the voltage
• Reduce the template concentration or use less sample
Possible Cause(s) Recommended Action

239DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: with high average signal intensity values
Secondary peaks under each
peak
Electropherogram
Ave Signal Intensity values are too high for data
from a 3100 instrument
Annotation
Possible Cause(s) Recommended Action
from the instrument. See page 197 for valid ranges.
Click the Annotation tab and examine the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Load less labeled sample by performing one of the
following:
• Dilute the resuspended product with Hi-Di
™
Formamide before loading onto the instrument
• Inject sample for less time
• Resequence the samples, using less template in the
sequencing reaction, especially if you use the BigDye
®
XTerminator
™
Purification Kit (see Tab le 8,
“Recommended DNA template quantities for cycle
sequencing,” on page 63).
Electrophoresis issue: Modified run module with
increased injection time was used.
Use an unmodified standard run module.

240 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: at the beginning of the sequence
Double peaks at the
beginning of the sequence
Single peaks for the
remaining sequence
Electropherogram
See also page 224 for the
raw data view
Possible Cause(s) Recommended Action
product is used as a sequencing template. Caused by the
formation of primer-dimers during the PCR reaction. The
primer-dimers anneal and are filled in to create short, non-
template PCR products.
If the sequence within the region affected by the primer-
dimer sequence is important, either:
• Redesign the PCR primers to eliminate the sequences
that allow primer-dimer formation
or
• Use a “hot start” PCR enzyme to inhibit primer-dimer
formation
More than 1 PCR product is present in the PCR reaction. Re-examine the sequence for primer site homology.
Redesign as necessary
More than 1 priming site (either upstream or downstream)
on the sequencing template.

241DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: at the beginning of the sequence (bisulfite conversion)
Electropherogram
Possible Cause(s) Recommended Action
product is used as a sequencing template. Caused by the
formation of primer-dimers during the PCR reaction. The
primer-dimers anneal and are filled in to create short, non-
template PCR products.
If the sequence within the region affected by the primer-
dimer sequence is important, use M13 tails with both
forward and reverse primers and either:
• Redesign the PCR primers to eliminate the sequences
that allow primer-dimer formation
or
• Use a “hot start” PCR enzyme to inhibit primer-dimer
formation

242 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: specific peaks under specific bases
C peaks under every peak
Electropherogram
Possible Cause(s) Recommended Action
Electrophoresis issues:
Poor or incorrect instrument spectral calibration.
Inspection of the raw data shows all secondary peaks
directly under primary peaks.
Perform a new spectral calibration run. Follow the
procedures in your instrument user guide. Then run your
samples again.
Poor quality matrix (310 instruments only). Create a new matrix file.
exceed the range used for spectral calibration because
too much template was used.
Click the Annotation tab and examine the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Load less labeled sample by performing one of the
following:
• Dilute the resuspended product with Hi-Di
™
Formamide before loading onto the instrument
• Inject sample for less time
• Resequence the samples, using less template in the
sequencing reaction, especially if you use the BigDye
®
XTerminator
™
Purification Kit (see Tab le 8,
“Recommended DNA template quantities for cycle
sequencing,” on page 63).

243DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: specific peaks under specific bases
C peaks under many
peaks using the ABI
basecaller
Electropherogram
Electropherogram
Clean sequence using the
KB basecaller
Possible Cause(s) Recommended Action
Data analysis issue: Using the ABI basecaller when
analyzing sequences for bisulfite-treated DNA. Bisulfite
treatment of DNA for methylation studies should convert
all unmethylated Cs to uracil, so the sequence should
contain very few C peaks. However, during sequence
analysis, the analysis software overcalibrates for the
absence of C peaks.
Use the KB basecaller to analyze sequences for bisulfite-
treated DNA.

244 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: peaks under peaks throughout (bisulfite conversion)
Electropherogram
Possible Cause(s) Recommended Action
conversion, indicated by the
presence of Cs (blue) not
adjacent to Gs (black). A C at a non-CpG position serves
as an internal control for complete bisulfite conversion.
Incomplete bisulfite conversion may be due to:
•Impure gDNA
• Too much gDNA
• Inadequate denaturation of gDNA prior to bisulfite
conversion
1. Check DNA quantitation and quality (page 44 and 45).
2. Repeat the bisulfite conversion
3. Repeat the sequencing.

245DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: double sequence (n+1 or n-1) throughout
n− 1 sequence
Electropherogram
Possible Cause(s) Recommended Action
Contamination of the PCR primer with n+1 or n-1 primer. Use the primers with a different template. If the problem
persists, resynthesize the primers before repeating the
experiment.
Contamination of the sequencing primer with n+1 or n-1
sequencing primer.
Sequencing primer contains a run of identical
nucleotides, especially 4 or more Gs.
Design new sequencing primers, avoiding runs of
identical nucleotides, especially 4 or more Gs.
Homopolymer at the beginning of the sequence. See page 246.

246 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: after a homopolymer or repeated sequence
Double peaks after a
homopolymeric region
Electropherogram
Possible Cause(s) Recommended Action
amplification and/or cycle sequencing. Stutter is most
common in any homopolymeric region greater than 2
bases. It can also be seen with simple repeated DNA
sequences. The results are worse when the stutter occurs
during PCR amplification.
It is thought that stutter occurs when a partially extended
primer and template dissociate, then reanneal improperly
before extension continues. Partially extended primers
and templates commonly dissociate during the reaction,
but if they reanneal with complete fidelity, the reaction
produces only one product. Improper annealing results in
one or more products that are represented in the
sequencing results.
If stutter occurs during PCR amplification, little can be
done to correct the problem, except using anchored
sequencing primers.
If stutter occurs during cycle sequencing:
• Try using dRhodamine terminators. They have been
shown to be less prone to produce stutters,
specifically with poly-T regions.
• Some customers have found that they can get past
poly(A) regions using a mixture of oligo dT
18
primers
with either a C, A, or G as the 3′ terminal dinucleotide
or 2-base anchors.

247DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: after a homopolymer or repeated sequence (bisulfite
sequencing)
.
Double sequence
after a homopolymer
region
Electropherogram
Region with repeating As
Possible Cause(s) Recommended Action
amplification and/or cycle sequencing. Stutter is most
common in any homopolymeric region greater than 2
bases. It can also be seen with simple repeated DNA
sequences. The results are worse when the stutter occurs
during PCR amplification.
It is thought that stutter occurs when a partially extended
primer and template dissociate, then reanneal improperly
before extension continues. Partially extended primers
and templates commonly dissociate during the reaction,
but if they reanneal with complete fidelity, the reaction
produces only one product. Improper annealing results in
one or more products that are each represented in the
sequencing results.
If stutter occurs during PCR amplification, little can be
done to correct the problem, except using anchored
sequencing primers.
If stutter occurs during cycle sequencing:
• Try using dRhodamine terminators. They have been
shown to be less prone to produce stutters,
specifically with poly(T) regions.
• Some customers have found that they can get past
poly(A) regions using a mixture of oligo dT
18
primers
with either a C, A, or G as the 3′ terminal dinucleotide
or 2-base anchors.
• Avoid stretches with > 8 As or Ts.
• Use BigDye
®
Terminator Ready Reaction Mix at full
strength.
• Use AmpliTaq Gold polymerase.

248 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Double peaks: double sequence after clean sequence
SeqScape Software detects HIM in the forward
and reverse strands
SeqScape Software Project View
Electropherogram
Double sequence
after clean
sequence
Possible Cause(s) Recommended Action
Heterozygous indel mutation (HIM). Obtain forward and reverse sequence data and assemble
using SeqScape or Variant Reporter
™
Software.
• SeqScape Software lists HIMs in the Mutations
Report. Review the mutation by clicking the Base
Change in the Mutations Report to view the mutation
in the Project view.
• Variant Reporter Software lists HIMs in the Project
Summary Report.

249DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Resolution loss: at beginning of run
Possible Cause(s) Recommended Action
XTerminator
™
Solution or premix exposed to temperature
over 25 °C.
• Use appropriate adapter for vortexer
• Make sure plate does not heat up during vortexing
step
BigDye XTerminator Purification Kit reagents past their
expiration date.
• Verify expiration dates on reagents and discard if
expired
• Store XTerminator Solution at 4 °C
• Store SAM Solution at room temperature

250 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Resolution loss: in the middle of the run
Loss of peak resolution in
the middle of the run
Electropherogram
Possible Cause(s) Recommended Action
Electrophoresis issues:
Migration of a contaminant or microbubbles through the
capillary during electrophoresis.
Run the sample again.
Syringes, polymer block, or septa contaminated with
chemicals during cleaning.
1. Perform a water wash through the polymer delivery
system, using the Data Collection Software wizard.
2. Replace polymer, buffer, and water/waste with fresh
materials.
3. Run the sample again.
Incomplete replacement of polymer between runs. Check the polymer delivery system for leaks, looking for
residue in and around the polymer block area; check the
pin valve for signs of arcing on the tip; check for polymer
in the anode buffer jar. If you see evidence of a leak,
retighten, then run the sample again. If the leaking
persists, contact Applied Biosystems to arrange a service
engineer visit.

251DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Resolution loss: gradual early loss
Raw Data
Electropherogram
Raw Data (zoomed in)
Zooming in on raw
data (circled above)
shows broad peaks
Resolution loss
marked by broad
peaks
Possible Cause(s) Recommended Action
Electrophoresis issues:
Capillary array degrading. 1. Perform a water wash through the polymer delivery
system, using the Data Collection Software wizard.
2. Replace the capillary/array.
3. Run a sequencing standard.
4. If the problem persists, replace reagents, then run your
samples again.
Samples degraded because they sat in the instrument too
long, >48 hours.
Prepare additional sample for electrophoresis, referring to
“Minimum Sample Volume” on page 122. Then, run the
samples again.
Expired or old reagents: polymer, Hi-Di
™
Formamide,
buffer, or water.
Replace the reagent, then run your samples again.

252 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
Electrophoresis source is faulty, resulting in unstable
current.
Contact Applied Biosystems to arrange a service
engineer visit.
Extension products purified using bead-based kits were
injected without removing the magnetic beads. The beads
may interfere with the extension products during injection
and cause overloading or other injection anomalies.
Remove magnetic beads before loading the sample.
Capillaries overloaded with sequencing product. Click the Annotation tab and examine the Ave Signal
Intensity. Excessive signal:
• 3730/3730xl instruments: >10,000 rfus
• 310 and 31XX instruments: >1000 rfus
Load less labeled sample by performing one of the
following:
• Dilute the resuspended product with Hi-Di Formamide
before loading onto the instrument
• Inject sample for less time
• Resequence the samples, using less template in the
sequencing reaction (especially if you use the BigDye
®
XTerminator
™
Purification Kit) (see Tab l e 8,
“Recommended DNA template quantities for cycle
sequencing,” on page 63).
Blending Ready Reaction Mixes from dGTP BigDye
terminator kits with BigDye terminator vx.1 kits.
Do not use blended Ready Reaction Mixes of dGTP
BigDye Terminator kits and BigDye Terminator vx.1 kits in
these cases.
Use of non-Applied Biosystems reagents. 1. Perform a water wash on all components of the
system using the wizard in Data Collection Software.
2. Replace reagents with Applied Biosystems products.
Note: the performance of non-Applied Biosystems
reagents cannot be guaranteed.
Possible Cause(s) Recommended Action

253DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting
High quality sequence in unassembled category in SeqScape Software
Gray sequence is in the unassembled category
High quality data
Possible Cause(s) Recommended Action
SeqScape or Variant Reporter
™
Software detects no
similarity between the sample sequence and the
reference sequence. The gray sequence indicates that the
trimming of the data to the reference sequence failed.
Make sure that the sample is included in the right project.
Incorrect sample identification when a sample belonging
to another project was imported.

254 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8 Troubleshooting

DNA Sequencing by Capillary Electrophoresis Chemistry Guide 255
Troubleshooting Index
A
annotation tab, reviewing 195
artifacts, reviewing for 194
average signal intensity
high values 204, 212, 223, 237, 240, 250
low value for C 229
reviewing 195
B
base spacing
reviewing 195
basecaller
appropriate location for file 193
verifying correct one is used 193
basecalling
mixed bases not called correctly 202
stops 217
too many mixed bases called 203
baseline
negative 214, 215
reviewing 194
waterfall symptom 216
BDX Update utility 208
BigDye
®
terminators
irregular C peaks symptom 229
irregular G peaks symptom 231
BigDye
®
XTerminator
™
Purfication Kit
BDX Update utility 208
symptom of incorrect run module 223
bisulfite sequencing
broad peaks 234
double peaks after homopolymer 245
double peaks at the beginning 239
peaks under peaks throughout 242
blobs in electropherogram 228
C
C peaks, irregular 229
compressions, of peaks 233
controls
troubleshooting using 192
current during run, reviewing 196
D
data analysis
PCR stop point 217
reviewing information 194
reviewing settings 193
settings on annotation tab 195
when mobility file is incorrect 227
with incorrect mixed bases settings 202
with incorrect quality threshold setting 202
Data Collection Software
settings on Annotation tab 195
data start point, reviewing 194
dGTP BigDye
®
terminators
peak compression symptom 233
DNA quality
troubleshooting 197
DNA quantity
troubleshooting 197
DNA template
difficult to sequence 218
double peaks
after homopolymer or repeated sequence 244, 245
double sequence after clean sequence 246
n+1 or n-1 sequence throughout 242
peaks under peaks throughout 235
specific peaks under specific bases 240, 241
with high average signal intensity values 237
dye degradation
symptom of 229, 231, 232
E
electropherogram
example 193
reviewing 193
EPT tab, reviewing 196
examples
electropherogram 193
EPT tab 196
raw data 194
troubleshooting symptoms 199
G
G peaks, irregular 231

256 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8
H
heterozygous indel mutation (HIM) detection 246
homopolymeric regions
troubleshooting sequencing of 204, 232, 236, 244,
245
I
indel, detection of 246
instrument run
reviewing 194
M
M13mp18 control
troubleshooting using 192
matrix file
appropriate location for 193
verifying correct one used 193
mixed bases
not called correctly 202
too many called 203
mobility file
appropriate location for 193
verifying correct one used 193
N
n+1 or n-1 sequence throughout 242
P
PCR stop point 217
peak heights, reviewing in raw data 194
peak symptoms
blobs 228
compressed peaks 233
double peaks 237, 238, 240, 241, 242, 244, 246
irregular C peaks 229
irregular G peaks 231
large peaks 228
missing C peaks 229
n+1 or n-1 sequence throughout 242
peaks under peaks throughout 235
resolution loss 248, 249
shoulders 229, 232
spikes 224, 225
split peaks 223
unevenly spaced peaks 227
pGEM-3Zf(+) control
troubleshooting using 192
poly(A) regions, sequencing 245
poly(T) regions, sequencing 245
polymer
problems caused by crystals 224
problems caused by heating 224, 225
power during run, reviewing 196
primers
primer-dimer formation 222
reviewing design of 198
R
raw data
example of high quality 194
reviewing 194
read length, reviewing 194
Ready Reaction Mix
blended 232, 250
improper storage 206
insufficient quantity 205, 209, 218, 219, 220, 221
reptation peak 226
resolution
loss of 212, 248, 249
run. See instrument run.
S
Sample Manager
example 193
red spacing value in 201
SeqScape
®
Software
detection of HIM using 246
high quality sequence in unassembled category
symptom 251
Sequencing Analysis Software
red spacing value in 201
reviewing data analysis settings 193
reviewing run and analysis information 194
sequencing standards
troubleshooting using 192
shoulders
on all peaks 232
on C peaks 229
signal symptoms
excessive signal 223
low signal 205, 207, 209
negative baseline 214, 215
no signal 205, 207
signal starts later than expected 211, 212
ski slope profile 221
spike 224, 225, 226
sudden drop in signal 217, 218, 219
top-heavy data 220, 221, 222, 223
waterfall baseline 216
signal/noise
reviewing 195
ski slope profile 221
spacing of peaks, troubleshooting 227
spacing value, red 201
spectral pull-up 235
spikes

257DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8
at end of run 226
four-color 224
one-color 225
split peaks 223
stutter 204, 232, 236, 244, 245
symptoms
blobs in electropherogram 228
double peaks 235, 237, 238, 240, 241, 242, 244,
246
excessive signal 223
high quality sequence in unassembled category 251
irregular C peaks 229
irregular G peaks 231
large peaks 228
low signal 205, 207, 209
missing C peaks 229
mixed bases not called correctly 202
n+1 or n-1 sequence throughout 243
negative baseline 214, 215
no signal 205, 207
peak compressions 233
peak shoulders 232
peaks under specific bases 240, 241
peaks unevenly spaced 227
resolution loss 212, 248, 249
shoulders on peaks 229
signal drops suddenly 217, 218, 219
signal starts later than expected 211, 212
ski slope profile 221
spikes 224, 225
, 226
sp
lit peak
s 223
table of 199
too many mixed bases called 203
top-heavy data 220, 221, 222, 223
waterfall baseline 216
T
temperature of run
reviewing 196
top-heavy data 220, 221, 222, 223
troubleshooting
DNA quantity and quality, reviewing 197
EPT tab 196
overview 192
primer design, reviewing 198
symptoms table 199
workflow 192
V
voltage
reviewing 196
W
waterfall baseline 216
workflows
troubleshooting 192

258 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Chapter 8

Part Number 4305080 Rev. C 05/2009

261DNA Sequencing by Capillary Electrophoresis Chemistry Guide
A
Product Information A
This chapter covers:
Peak Color/Base Relationships . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .262
Control Sequences. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .263

262 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix A Product Information
Peak Color/Base Relationships
Peak Color in
Raw Data
Because sequencing chemistries are developed to produce the most uniform signal in
the analyzed data, the different chemistries may have different color (dye)/base
relationships.
Peak Color in
Electrophero-
grams
All Applied Biosystems sequencing analysis software compensate for the different
color/base relationships when the correct mobility file is used (see “Run Parameters
for Instrument and Analysis Protocols” on page 151). The software always displays
analyzed data in the electropherogram as shown in Table 39.
Table 38 Color/base relationships in raw data
Base
Peak Color in Raw Data
BigDye
®
Term in at or s
dGTP BigDye
Te r mi na to r s
BigDye
Primers
dRhodamine
Te r mi na to r s
A green green green green
C red red blue black
G blue blue black blue
T black black red red
Table 39 Color/base relationships in electropherograms
Base
Peak Color in
Electropherogram
Agreen
Cblue
G black
Tred

263DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix A Product Information
Control Sequences
M13 Control
Primers
All Applied Biosystems dye terminator cycle sequencing kits include M13 control
primers at 0.8 pmol/μL.
M13 Forward Primer Sequence
5´TGTAAAACGACGGCCAGT 3´
M13 Reverse Primer Sequence
5´CAGGAAACAGCTATGACC 3´
pGEM-3Zf(+)
Sequence
All Applied Biosystems DNA sequencing kits provide pGEM control DNA at
0.2 μg/μL. The pGEM-3Zf(+) sequence below is the sequence of the 1000 bases that
follow the M13 forward primer (GenBank accession number X65306).
20 40 60
GAATTGTAAT ACGACTCACT ATAGGGCGAA TTCGAGCTCG GTACCCGGGG ATCCTCTAGA
80 100 120
GTCGACCTGC AGGCATGCAA GCTTGAGTAT TCTATAGTGT CACCTAAATA GCTTGGCGTA
140 160 180
ATCATGGTCA TAGCTGTTTCCTGTGTGAAA TTGTTATCCG CTCACAATTCCACACAACAT
200 220 240
ACGAGCCGGA AGCATAAAGT GTAAAGCCTG GGGTGCCTAA TGAGTGAGCTAACTCACATT
260 280 300
AATTGCGTTG CGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCC AGCTGCATTA
320 340 360
ATGAATCGGCCAACGCGCGG GGAGAGGCGG TTTGCGTATT GGGCGCTCTT CCGCTTCCTC
380 400 420
GCTCACTGAC TCGCTGCGCT CGGTCGTTCGGCTGCGGCGA GCGGTATCAG CTCACTCAAA
440 460 480
GGCGGTAATA CGGTTATCCA CAGAATCAGG GGATAACGCA GGAAAGAACA TGTGAGCAAA
500 520 540
AGGCCAGCAA AAGGCCAGGA ACCGTAAAAA GGCCGCGTTG CTGGCGTTTT TCCATAGGCT
560 580 600
CCGCCCCCCTGACGAGCATC ACAAAAATCGACGCTCAAGT CAGAGGTGGC GAAACCCGAC
620 640 660
AGGACTATAA AGATACCAGG CGTTTCCCCC TGGAAGCTCC CTCGTGCGCT CTCCTGTTCC
680 700 720
GACCCTGCCG CTTACCGGAT ACCTGTCCGCCTTTCTCCCTTCGGGAAGCG TGGCGCTTTC
740 760 780
TCATAGCTCA CGCTGTAGGT ATCTCAGTTC GGTGTAGGTC GTTCGC TCCAAGCTGGGCTG
800 820 840
TGTGCACGAA CCCCCCGTTC AGCCCGACCG CTGCGCCTTA TCCGGTAACTATCGTCTTGA
860 880 900
GTCCAACCCG GTAAGACACGACTTATCGCC ACTGGCAGCAGCCACTGGTA ACAGGATTAG
920 940 960
CAGAGCGAGG TATGTAGGCG GTGCTACAGA GTTCTTGAAG TGGTGGCCTA ACTACGGCTA
980 1000
CACTAGAAGG ACAGTATTTG GTATCTGC GC TCTGCTGAAG

264 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix A Product Information
Sequencing
Standard
Sequence
This section contains the sequence for the following sequencing standards:
•BigDye
®
Terminator v1.1 Sequencing Standard
•BigDye
®
Terminator v3.1 Sequencing Standard
• dRhodamine Terminator Cycle Sequencing Standard
You can obtain the sequence of the first 1000 bases using GenBank accession
number AY390769.
20 40 60
AATTCCCTGC AGGCGTGGCTGCAGCCTGGT TATGATTACT GTTAATGTTG CTACTACTGC
80 100 120
TGACAATGCTGCTGCTGCTT CTCCTCACTG TCTCCACTTCCTTGAACAAT GCGCCGTCAT
140 160 180
GCTTCTTTTG CCTCCCGCTG CTCCAGAAAG CTAGGCCGCA GATCAGAACC ACCACAGTC A
200 220 240
ATATCACCACCTTCCTCTTA TAGATTCGGA ATCTCATGAT AGGGGC TCAG CCTCTGTGCG
260 280 300
AGTGGAGAGA AGTTTGCAGG CGAGCTGAGG AGC AATTGCA GGTGATATGA TGTGCTCGGC
320 340 360
TCAAGAAGCGGGCCCGGAGA GGAAGAAGTC GTGCCGGGGC TAATTATTGG CAAAACGAGC
380 400 420
TCTTGTTGTA AACATTGATCCAACTGGAAT GTCACTAATG GCGAATCAAT ATTCCATAAG
440 460 480
GCATGATGGT TGCTCAGAGG CAGGAGAAGA GCAACGAATA CGATCCTATA AAAGATAAAA
500 520 540
CATAAATAAA CAGTCTTGAT TATATTCTGG GTATTAAAGCCACAATCAGA ACAAATATAT
560 580 600
GCTTTGTATC TTTTCTTGCC TTCTTC ATTA CCAACTGCTT CCGCGGCCAC ATTAAGAGAA
620 640 660
CTTGTGGTAA GATAAGAAGA TATTTTATTC GTTCTGCTGA CTTGCTGGAT GTCGGGAAAT
680 700 720
ATTCTGCATT TGATAAGAGG CGGTTAATTG CAGATATAAT TGGTAGTGAA AAGGGTCGTT
740 760 780
GCTATGGTCA CCGTGAAGCG AGTACAGCAG CACAAGAATG TGTGCCGTTC TCAGTTAATA
800 820 840
TTGTTTGAAT ATGGTAACCT GTTTTAGTCG GTTTAAAGGT AAGAAGATCTAACCAAAAAC
860 880 900
AACACTGCAG TGACTGATTG TAGTATTTAT TTTTTTACTT AATCTTAATT TTGGTGTAAA
920 940 960
CATCAACGGC GCACTTCAACCAATACTCCA ATGTTTTATCCATCGACATG ACGTTCGAGA
980 1000 1020
TAGGGTTGAG TGTTGTTCCA GTTTGGAACA AGAGTCCACT ATTAAAGAAC GTGGACTCCA
1040 1060 1080
ACGTCAAAGG GCGAAAAACC GTCTATC AGG GCGATGGCCC ACTACGTGAA CCATCACCCA
1100 1120 1140
AATCAAGTTT TTTGGGGTCG AGGTGCCGTA AAGCACTAAA TCGGAACCCT AAAGGGAGCC
1160 1180 1200
CCCGATTTAG AGCTTGACGG GGAAAGCCGG CGAACGTGGC GAGAAAGGAA GGGAAGAAAG

265DNA Sequencing by Capillary Electrophoresis Chemistry Guide
B
Parts List B
This chapter covers:
Kits and Reagents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .266
Instrument Parts. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .270
Software Products . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .278
Miscellaneous Documents. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .279

266 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
Kits and Reagents
BigDye
®
XTerminator
™
Purification Kits
BigDye
®
Terminator v1.1
Cycle
Sequencing Kits
BigDye
Terminator v3.1
Cycle
Sequencing Kits
Product
Approximate Number
of 20-μL Reactions
Part
Number
BigDye
®
XTerminator
™
Purification Kit (2-mL) 100 4376486
BigDye XTerminator Purification Kit (20-mL) 1,000 4376487
BigDye XTerminator Purification Kit (50-mL) 2,500 4376484
BigDye XTerminator Purification Kit (800-mL) 40,000 4376485
Product
Part
Number
BigDye
®
Terminator v1.1 Cycle Sequencing Kit (24 rxns) 4337449
BigDye
®
Terminator v1.1 Cycle Sequencing Kit (100 rxns) 4337450
BigDye
®
Terminator v1.1 Cycle Sequencing Kit (1000 rxns) 4337451
BigDye
®
Terminator v1.1 Cycle Sequencing Kit (5000 rxns) 4337452
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (1 mL) 4336697
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (25 mL) 4336699
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (230 mL) 4336701
BigDye
®
Terminator v1.1 Matrix Standard (3100-Avant, 3100) 4336824
BigDye
®
Terminator v1.1 Matrix Standards (310) 4336805
BigDye
®
Terminator v1.1 Sequencing Standard (3730, 3730xl) 4336799
BigDye
®
Terminator v1.1 Sequencing Standard (310, 3100-Avant), 3100 4336791
Product
Part
Number
BigDye
®
Terminator v3.1 Cycle Sequencing Kit (24 rxns) 4337454
BigDye
®
Terminator v3.1 Cycle Sequencing Kit (100 rxns) 4337455
BigDye
®
Terminator v3.1 Cycle Sequencing Kit (1000 rxns) 4337456
BigDye
®
Terminator v3.1 Cycle Sequencing Kit (5000 rxns) 4337457
BigDye
®
Terminator v3.1 Cycle Sequencing Kit (25000 rxns) 4337458
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (1 mL) 4336697
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (25 mL) 4336699

267DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
BigDye
Terminator dGTP
Cycle
Sequencing Kits
Note: 5 ✕ Sequencing Buffer for the BigDye Terminator dGTP Cycle Sequencing
Kit is not the same as BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer. Be sure to
use the correct buff
er.
dRhodamine
Terminator Cycle
Sequencing Kits
Note: 5 ✕ Sequencing Buffer for the dRhodamine Terminator Cycle Sequencing
Kit is not the same as BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer. Be sure to
use the correct buff
er.
BigDye
®
Terminator v1.1/v3.1 Sequencing Buffer (5 ✕ ) (230 mL) 4336701
BigDye
®
Terminator v3.1 Matrix Standards (310) 4336948
BigDye
®
Terminator v3.1 Matrix Standard (3100-Avant, 3100, 3130, 3130xl) 4336974
BigDye
®
Terminator v3.1 Sequencing Standard (310, 3100-Avant, 3100,
3130, 3130xl)
4336935
BigDye
®
Terminator v3.1 Sequencing Standard (3730, 3730xl) 4336943
Product
Part
Number
Product
Part
Number
5 ✕ Sequencing Buffer (1 tube) 4305605
5 ✕ Sequencing Buffer (9 tubes) 4305603
dGTP BigDye
®
Terminator v1.0 Cycle Sequencing Ready Reaction Kit (100
rxns)
4307175
dGTP BigDye
®
Terminator v3.0 Cycle Sequencing Ready Reaction Kit (100
rxns)
4390229
Product
Part
Number
5 ✕ Sequencing Buffer (1 tube) 4305605
5 ✕ Sequencing Buffer (9 tubes) 4305603
dRhodamine Dye Terminator Cycle Sequencing Ready Reaction Kit (1000
rxns)
403045
dRhodamine Dye Terminator Cycle Sequencing Ready Reaction Kit (5000
rxns)
4303143
dRhodamine Matrix Standards Kit (310) (10 rxns) 403047
dRhodamine Matrix Standards Kit (310) (4 tubes) 403046
Long Read dRhodamine Terminator Cycle Sequencing Standard (1 tube) 4303120

268 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
BigDye Primer
Cycle
Sequencing Kits
Custom Primers
You can obtain custom primers from the Applied Biosystems Custom
Oligonucleotide Synthesis Service. See page 40 for instructions.
Matrix Standards
Sequencing
Standards
Product
Part
Number
BigDye
®
Primer Cycle Sequencing Ready Reaction Kit, − 21 M13 (100 rxns
and protocol)
403051
BigDye
®
Primer Cycle Sequencing Ready Reaction Kit, − 21 M13 (5000 rxns) 403049
BigDye
®
Primer Cycle Sequencing Ready Reaction Kit, M13 REV (100 rxns
and protocol)
403052
BigDye
®
Primer Cycle Sequencing Ready Reaction Kit, M13 REV (5000 rxns) 403050
Standard
Part
Number
BigDye
®
Terminator v1.1 Matrix Standard (3100-Avant, 3100) 4336824
BigDye
®
Terminator v1.1 Matrix Standards (310) 4336805
BigDye
®
Terminator v3.1 Matrix Standard (3100-Avant, 3100, 3130, 3130xl) 4336974
BigDye
®
Terminator v3.1 Matrix Standards (310) 4336948
dRhodamine Matrix Standards Kit (310) (4 tubes) 403046
dRhodamine Matrix Standards Kit (310) (10 rxns) 403047
Matrix Standard Set DS-01, dROX, dTAMRA, dR6G, dR110 4305609
Standard
Part
Number
BigDye
®
Terminator v1.1 Sequencing Standard (3730, 3730xl) 4336799
BigDye
®
Terminator v1.1 Sequencing Standard (310, 3100-Avant, 3100 4336791
BigDye
®
Terminator v3.1 Sequencing Standard (3730, 3730xl) 4336943
BigDye
®
Terminator v3.1 Sequencing Standard (310, 3100-Avant, 3100,
3130, 3130xl)
4336935
Long Read dRhodamine Terminator Cycle Sequencing Standard (1 tube) 4303120

269DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
Reagent Kit
Protocols
Plastics
Protocol
Part
Number
BigDye
®
Terminator v1.1 Cycle Sequencing Kit Protocol 4337036
BigDye
®
Terminator v3.1 Cycle Sequencing Kit Protocol 4337035
dGTP BigDye
®
Terminator Ready Reaction Kit With AmpliTaq
®
DNA
Polymerase, FS Protocol
4339924
dGTP BigDye
®
Terminator v3.0 Ready Reaction Cycle Sequencing Kit
Protocol
4307169
dRhodamine Terminator Cycle Sequencing Ready Reaction Kit With
AmpliTaq
®
DNA Polymerase, FS Protocol
4339921
BigDye
®
Primer Cycle Sequencing Ready Reaction Kit With AmpliTaq
®
DNA
Polymerase, FS Protocol
4339922
Product
Part
Number
MicroAmp
®
Fast 96-Well Reaction Plate, 0.1 mL 4346907
MicroAmp
®
Optical Film Compression Pad (5 per pack) 4312639
MicroAmp
®
Optical 384-Well Reaction Plate 4343370
MicroAmp
®
Clear Adhesive Films 4306311
MicroAmp
®
Optical 96-Well Reaction Plate N8010560
MicroAmp
®
Reaction Tube without Cap, 0.2-mL N8010533
Genetic Analyzer Sample Tubes, 0.5 mL 401957

270 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
Instrument Parts
Capillary
Consumables Product
Part
Number
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di
™
Formamide (25 mL) 4311320
3730/3730xl Capillary Consumables
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di Formamide (25 mL) 4311320
3730 36-cm capillary array 4331247
3730 50-cm capillary array 4331250
3730xl 36-cm capillary array 4331244
3730xl 50-cm capillary array 4331246
Applied Biosystems 3730/3730xl 10 ✕ Running Buffer With EDTA (500 mL) 4335613
POP-7
™
Polymer (one 28-mL bottle) 4363929
POP-7
™
Polymer (ten 28-mL bottles) 4363935
POP-7
™
Polymer (thirty 28-mL bottles) 4335611
96-well sample plates w/barcode 4306737
96-well sample plates, no barcode N8010560
96-well plate septa 4315933
384-well sample plates w/barcode 4309849
384-well plate septa 4315934
Heat Seal Film For Sequencing and Fragment Analysis Sample Plates 4337570
3130/3130xl Capillary Consumables
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di Formamide (25 mL) 4311320
3130 22-cm capillary array 4333463
3130 36-cm capillary array 4333464
3130 50-cm capillary array 4333466
3130 80-cm capillary array 4333465
3130xl 22-cm capillary array 4319898

271DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
3130xl 36-cm capillary array 4315931
3130xl 50-cm capillary array 4315930
3130xl 80-cm capillary array 4319899
POP-4
™
Polymer (3.5 mL) 4363752
POP-4
™
Polymer (7 mL) 4352755
POP-6
™
Polymer (3.5 mL) 4363783
POP-6
™
Polymer (7 mL) 4352757
POP-7
™
Polymer (3.5 mL) 4363785
POP-7
™
Polymer (7 mL) 4352759
3100-Avant Capillary Consumables
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di Formamide (25 mL) 4311320
Autosampler 96-well plate kit 4316471
Autosampler 384-well plate kit 4316472
3100 POP-6
™
Polymer (7 mL) 4316357
3100 POP-4
™
Polymer (7 mL) 4316355
96-well plate septa (20) 4315933
384-well plate septa (20) 4315934
3100 Instrument Consumable: Reservoir Septa (20) 4315932
3100-Avant 22-cm capillary array 4333463
3100-Avant 36-cm capillary array 4333464
3100-Avant 50-cm capillary array 4333466
3100-Avant 80-cm capillary array 4333465
3100 Capillary Consumables
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di Formamide (25 mL) 4311320
3100 Instrument Consumable: Reservoir Septa (20) 4315932
Lower Polymer Block, v1.0 638-3680
Product
Part
Number

272 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
3100 POP-4
™
Polymer (7 mL) 4316355
3100 POP-6
™
Polymer (7 mL) 4316357
3100 22-cm capillary array 4319898
3100 36-cm capillary array 4315931
3100 50-cm capillary array 4315930
3100 80-cm capillary array 4319899
310 Capillary Consumables
10 ✕ Genetic Analyzer Buffer With EDTA (25 mL) 402824
Hi-Di Formamide (25 mL) 4311320
310 capillaries, 47cm ✕ 50 μm i.d. (internally uncoated) (5 pkg.) 402839
310 capillaries, 61cm ✕ 50 μm i.d. (internally uncoated) (2 pkg.) 402840
48-tube sample tray kit 402867
96-tube sample tray kit 402868
Genetic analyzer 4.0-mL buffer vials (50) 401955
Genetic analyzer retainer clips (96-tube tray septa clips) (4 pkg.) 402866
Genetic analyzer 0.5-mL Sample tubes (500) 401957
Genetic analyzer septa for 0.5-mL sample tubes (500) 401956
Genetic analyzer septa strips (0.2-mL tube) (480) 4305547
POP-4
™
Polymer for 310 genetic analyzers (5 mL) 402838
POP-6
™
Polymer for 310 genetic analyzers (3 mL) 402837
Product
Part
Number

273DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
3730/3730xl
Instrument Parts
and Documents
Product
Part
Number
3730/3730xl Instrument Accessories
384-Well Plate Autosampler Installation Kit 4336134
384-Well Plate Base (Heat Seal) (4) 4334877
384-Well Plate Base (Septa Seal) (4) 4334874
384-Well Plate Retainer (Septa Seal) (4) 4334868
96-Well and 384-Well Plate Retainer (Heat Seal) (4) 4334865
96-Well Plate Autosampler Installation Kit 4336131
96-Well Plate Base (Heat Seal) (4) 4334875
96-Well Plate Base (Septa Seal) (4) 4334873
96-Well Plate Retainer (Septa Seal) (4) 4334869
Anode Buffer Jar, 67-mL, v2.0 625-2045
Array Ferrule Knob 628-3730
Array Ferrule Sleeves, Peek (4) 628-0165
Buffer, Water, and Waste Labels (4 sheets) 625-1195
Buffer, Water, and Waste Retainer (3) 625-0500
Cathode Buffer Plate Base 625-0504
Drip Tray 625-1088
Lower Polymer Block, v2.0 625-2040
PDP/Lower Block Interconnect Line, Radel, v2.0 (2) 625-2043
Polymer Bottle Cap and Line, Radel, v2.0 (2) 625-2047
Reservoir Cap (3) 625-0503
Reservoir, Buffer Water Waste (3) 625-0501
Water and Waste Plate Base (4) 625-0502

274 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
3130/3130xl
Instrument Parts
and Documents
3100-Avant
Instrument Parts
and Documents
Product
Part
Number
3130/3130xl Instrument Accessories
Array comb holders 628-0164
Array port plug 628-3776
Buffer reservoir, 16 mL 4358351
Buffer/water/waste reservoir 628-0163
Ferrule knob 628-3730
Ferrule sleeves 628-0165
Polymer bottle 4362387
Polymer tubing assembly 628-3732
Septa strip, buffer reservoir 4315932
3130/3130xl Instrument Documents
Applied Biosystems 3130/3130xl Genetic Analyzers Getting Started Guide 4352715
Applied Biosystems 3130/3130xl Genetic Analyzers Maintenance,
Troubleshooting, and Reference Guide
4352716
Applied Biosystems 3130/3130xl Genetic Analyzers Quick Reference Card 4362825
Applied Biosystems 3730/3730xl DNA Analyzers and 3130/3130xl Genetic
Analyzers AB Navigator Software Administrator Guide
4359472
Product
Part
Number
3100-Avant Instrument Accessories
Anode buffer reservoir jar 5402
96-well plate retainer (4) 4317241
96-well plate base (AB) (4) 4317237
384-well plate retainer (4) 4317240
384-well plate base (4) 4317236
3100-Avant Instrument Documents
ABI P
RISM
®
3100-Avant Genetic Analyzer User Guide 4333549
ABI P
RISM
®
3100 Genetic Analyzer and 3100-Avant Genetic Analyzer User
Reference Guide
4335393

275DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
3100 Instrument
Parts and
Documents
ABI PRISM
®
3100/3100-Avant Data Collection v2.0 User Guide 4347102
Product
Part
Number
Item
Part
Number
3100 Instrument Accessories
250-μL glass syringe (array-fill syringe) (Contains syringe, o-rings, and ferrule) 4304470
5.0-mL glass syringe (polymer reserve syringe) (Contains syringe, 0-rings,
and ferrule)
628-3731
96-well plate base (AB) (4) 4317237
96-well plate retainer (4) 4317241
96-well plate septa (20) 4315933
384-well plate base (4) 4317236
384-well plate retainer (4) 4317240
384-well plate septa (20) 4315934
Anode buffer reservoir jar 005402
Array calibration ruler 628-3214
Array ferrule knob 628-3730
Array ferrule sleeves, PEEK (4) 638-0165
Autosampler 384-well plate kit 4316472
Autosampler 96-well plate kit 4316471
Reservoirs (for buffer, water, and waste) 628-0163
Polymer block tubing assembly 628-3732
Syringe ferrule 005401
Syringe O-rings 221102
Drip tray, lower polymer block 628-3088
Drip tray, autosampler 628-3059
Array calibration ruler 628-3732
Array comb holders 628-3403
Array ferrule sleeves 628-0165
Array ferrule knob 628-3730

276 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
310 Instrument
Parts and
Documents
3100 Instrument Spare Parts
Drip tray, autosampler 628-3059
Lower polymer block drip tray 628-3088
Upper polymer block drip tray 628-3720
3100 Computer Accessories
ABI P
RISM
®
3100 Genetic Analyzer Operator Training CD 4322559
3100 Instrument Documents
ABI PRISM
®
3100 Genetic Analyzer Quick Start Guide For Sequencing 4315833
ABI P
RISM
®
3100 Genetic Analyzer and 3100-Avant Genetic Analyzer User
Reference Guide
4335393
ABI PRISM
®
3100 Genetic Analyzer User Guide 4334785
ABI P
RISM
®
3100/3100-Avant Data Collection v2.0 User Guide 4347102
Item
Part
Number
Item
Part
Number
310 Instrument Accessories
0.5-mL sample tray 5572
1.0-mL glass syringe 4304471
2.5-μL glass syringe 604042
96-Well tray adaptor 4305051
Anode buffer reservoir jar 5402
Basic bundle for Applied Biosystems 310 Genetic Analyzer 4327421
Capillary fitting 5404
Filter, air 10 ✕ 20 ✕ 7/8 201356
FTG, 1/4-28 TFE Syringe Plug 310 110319
FTG, Capillary Plug 7023
G5v2 Module CD 4339037
Gel Block Waste Vial 603796
Genetic Analyzer Capillary Cutters (2) 401958

277DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
O-Ring, 5/64ID ✕ 13/64OD ✕ 1/16BUNA 221100
O-Ring, 5/8 ✕ 1/2 ✕ 1/16 Silicone 221085
O-Ring, 7/8ID ✕ 0.7 BUNA-N 221101
Syringe Ferrule, Bushing Seal 5401
Syringe Fitting Assembly 604071
Thermal Tape 310021
Waste Fitting Assembly 604076
310 Instrument Documents
ABI PRISM
®
310 Genetic Analyzer User Guide 4317588
Item
Part
Number

278 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
Software Products
Primer Design
Sequence
Scanner Software
Sequence Scanner software can be downloaded for free from the Applied Biosystems
web site at www.appliedbiosystems.com/sequencescanner.
Sequencing
Analysis Software
Variant Reporter
™
Software
SeqScape
®
Software
MicroSeq
®
ID
Analysis Software
Product
Part
Number
Methyl Primer Express
®
Software
‡
‡ Can be downloaded for free from the Applied Biosystems web site.
4376041
Product
Part
Number
Sequencing Analysis Software v5.3.1 (single-user) 4360967
Product
Part
Number
Variant Reporter™ Software v1.0, 30-Day Demo 4385270
Variant Reporter™ Software v1.0, Initial License 4385261
Product
Part
Number
SeqScape
®
Software version 2.5, 45-Day Demo (5 users) 4327099
SeqScape
®
Software version 2.5, Initial License 4327091
Product
Part
Number
MicroSeq
®
ID 16S rDNA 500 Library v2.0 4371294
MicroSeq
®
ID 16S rDNA Full Gene Library v2.0 4371295
MicroSeq
®
ID Fungal Gene Library v2.0 4371296
MicroSeq
®
ID Microbial Identification Software v2.0 Demo 4371297
MicroSeq
®
ID Microbial Identification Software v2.0 bundle (MSID v2.0
analysis software with bacterial and fungal libraries)
4371298
MicroSeq
®
ID Microbial Identification Software v2.0 Upgrade Bundle from
MicroSeq
®
Mac Software
4371300
MicroSeq
®
ID Microbial Identification Software v2.0 4371431

279DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List
Documents
Miscellaneous Documents
The following documents are cited in the text.
Product
Part
Number
Applied Biosystems MicroSeq
®
ID Analysis Software Version 2.0 Getting
Started Guide, Rev B
4364623
Applied Biosystems Variant Reporter™ Software Version 1.0 Getting Started
Guide
4376590
Applied Biosystems DNA Sequencing Analysis Software v5.1 User Guide 4346366
Applied Biosystems SeqScape
®
Software v2.5 User Guide 4359442
Applied Biosystems Sequencing Analysis Software Version 5.2 Quick
Reference Card
4359446
KB
™
Basecaller Frequently Asked Questions: KB Basecaller Software v1.4 4362968
Sequencing Analysis Software v5.2 User Bulletin 4358355
Document
Part or
Publication
Number
A Workflow for Obtaining High Quality Sequencing Data from Bacterial
Artificial Chromosome (BAC) DNA
107AP05-01
Advances in Fast PCR Contribute to a Fast Resequencing Workflow 104AP04-01
BigDye
®
Terminator v3.1 Cycle Sequencing Kit Protocol 4337035
BigDye
®
XTerminator
™
Purification Kit Protocol 4374408
dGTP BigDye
®
Terminator Ready Reaction Kit Protocol 4307169
dGTP BigDye
®
Terminator v3.0 Ready Reaction Cycle Sequencing Kit
Protocol
4390038
How to Order Primers and Probes (Publication 132GU01-01) 113920
Introducing New 96-Well Fast Plate Adapters for Applied Biosystems
Capillary Electrophoresis Systems User Bulletin
4370890
Precipitation Methods to Remove Residual Dye Terminators from
Sequencing Reactions User Bulletin
4304655

280 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Appendix B Parts List

281DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Bibliography
Literature Cited in
Te x t
Ausubel, F.M., Brent, R., Kingstin, R.E., Moore, D.D., Seidman, J.G., Smith, J.A.,
and Struhl, K., eds. 1998. Current Protocols in Molecular Biology. John Wiley and
Sons: New York, NY, p. A.3.0.2.
Bartlett, J., and Stirling, D., eds. 2003. PCR Protocols, 2nd ed. Totowa (NJ): Humana
Press, 556 pp.
Beutler, E., Gelbart, T., and Kuhl, W. 1990. Interference of heparin with the
polymerase chain reaction. BioTechniques 9(2): 166.
Boyd, V. L., Zon, G., 2004. Bisulfite conversion of genomic DNA for methylation
analysis: protocol simplification with higher recovery applicable to limited samples
and increased throughput, Anal. Biochem. 326(2):278-80.
Hoefer, Inc. 1993. Hoefer TKO 100 Mini-fluorometer Operator’s Manual:
20788/Rev C/3-05-93.
Holodniy, M., Kim, S., Katzenstein, D., Konrad, M., Groves, E., Merigan, T.C. 1991.
Inhibition of human immunodeficiency virus gene amplification by heparin. J. Clin.
Microbiol. 29(4): 676-679
Innis, M.A., and Gelfand, D.H. 1990. Optimization of PCRs. In PCR Protocols: A
Guide to Methods and Applications, ed. Innis, M.A., Gelfand, D.H., Sninsky, J.J., and
White, T.J. Academic Press: San Diego, CA, pp. 3–12.
Jackson R.L., Busch S.J. and Cardin A.D., 1991. Glycosaminoglycans: molecular
properties, protein interactions, and role in physiological processes. Physiol.
Reviews, 71, 481-539.
Kornberg, A., and Baker, T. 2005. DNA Replication, 2nd ed. New York (NY):
University Science Books. 932 pp.
Marra, M., Weinstock, L.A., and Mardis, E.R. 1996. End sequence determination
from large insert cloning using energy transfer fluorescent primers. Genome Res.
6: 1118–1122.
Meissner, A., Gnirke, A., Bell, G.W., Ramsahoye, B., Lander, E.S., Jaenisch, R.
2005. Reduced representation bisulfite sequencing for comparative high-resolution
DNA methylation analysis. Nucleic Acids Res. 33(18):5868-5877
QIAGEN. 1998. QIAGEN Guide to Template Purification and DNA Sequencing, 2nd
ed. Hilden, Germany. 66 pp.
QIAGEN. 1999-2001. QIAGEN Genomic DNA Handbook. 68 pp.
QIAGEN. 2002. QIAprep M13 Handbook. 32 pp.
Saha, S. et al. 2002. Using the transcriptome to annotate the genome. Nature
Biotechnol. 20(5):508-512.
Sambrook, J. and Russell, D. 2001. Molecular Cloning: A Laboratory Manual, 3rd
ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. 2344 pp.

282
Bibliography
DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Sambrook, J., Maniatis, T., and Fritsch, E.F. 1989. Molecular Cloning: A Laboratory
Manual, 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press.
1659 pp.
Sanger, F., Donelson, J.E., Coulson, A.R., Kösse,l H., Fischer D. 1974.
Determination of a nucleotide sequence in bacteriophage f1 DNA by primed
synthesis with DNA polymerase. J Mol Biol. 90(2): 315-33.
Sanger, F., Nicklen, S., and Coulson, A.R. 1977. DNA sequencing with chain-
terminating inhibitors. Proc. Natl. Acad. Sci (USA) 74: 5463–5467.
Wallace, A.J., Wu, C.L., Elles, R.G. 1999. Meta-PCR: a novel method for creating
chimeric DNA molecules and increasing the productivity of mutation scanning
techniques. Genet. Test. 3(2): 173-83
Warnecke P.M., Stirzaker C., Song J., Grunau C., Melki J.R., and Clarke S.J. 2002.
Identification and resolution of artifacts in bisulfite sequencing. Methods
27: 101–107.
Werle, E., Schneider, C., Renner, M., Volker, M., and Fiehn, W. 1994. Convenient
single-step, one tube purification of PCR products for direct sequencing. Nucleic
Acids Res. 22: 4354–4355.
Velculescu, V.E., Zhang, L., Vogelstein, B., Kinzler, K.W. 1995. Serial analysis of
gene expression. Science. 270(5235): 484-487.
Web Sites
Centre National de Séquençage (CNS, or Génoscope): www.cns.fr
QIAGEN: www.qiagen.com
Sanger Centre: www.sanger.ac.uk
The Association of Biomolecular Resource Facilities DNA Sequencing Research
Group: www.abrf.org
University of Oklahoma Advanced Center for Genome Technology (ACGT):
www.genome.ou.edu
University of Washington Genome Center: www.genome.washington.edu/uwgc
Washington University School of Medicine Genome Sequencing Center (GSC):
genome.wustl.edu/gsc
International Union of Biochemistry/International Union of Pure and Applied
Biochemistry (IUB/IUPAC): www.chem.qmw.ac.uk/iubmb/misc/naseq.html#300.
Additional
References
Barr, P.J., Thayer, R.M., Laybourn, P., Najarian, R.C., Seela, F., and Tolan, D.R. 1986.
7-deaza-2´-deoxyguanosine-5´-triphosphate: enhanced resolution in M13 dideoxy
sequencing. BioTechniques 4: 428–432.
Baskaran, N., Kandpal, R.P., Bhargava, A.K., Glynn, M.W., Bale, A., and Weissman,
S.M. 1996. Uniform amplification of a mixture of deoxyribonucleic acids with
varying GC content. Genome Res. 6: 633–638.
Burgett, S.G., and Rosteck, P.R., Jr. 1994. Use of dimethyl sulfoxide to improve
fluorescent, Taq cycle sequencing. In Automated DNA Sequencing and Analysis, ed.
Adams, M.D., Fields, C., and Venter, J.C. Academic Press: San Diego, CA,
pp. 211–215.

283
Bibliography
DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Devine, S.E., and Boeke, J.D. 1994. Efficient integration of artificial transposons
into plasmid targets in vitro: a useful tool for DNA mapping, sequencing, and
functional analysis. Nucleic Acids Res. 22: 3765–3772.
Devine, S.E., Chissoe, S.L., Eby, Y., Wilson, R.K., and Boeke, J.D. 1997. A
transposon-based strategy for sequencing repetitive DNA in eukaryotic genomes.
Genome Res. 7: 551–563.
Fernandez-Rachubinski, F., Eng, B., Murray, W.W., Blajchman, M.A., and
Rachubinski, R.A. 1990. Incorporation of 7-deaza dGTP during the amplification
step in the polymerase chain reaction procedure improves subsequent DNA
sequencing. DNA Seq. 1: 137–140.
Henke, W., Herdel, K., Jung, K., Schnorr, D., and Loening, S.A. 1997. Betaine
improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res.
25: 3957–3958.
Innis, M.A. 1990. PCR with 7-deaza-2´-deoxyguanosine triphosphate. In PCR
Protocols: A Guide to Methods and Applications, ed. Innis, M.A., Gelfand, D.H.,
Sninsky, J.J., and White, T.J. Academic Press: San Diego, CA, pp. 54–59.
Khan, A.S., Wilcox, A.S. , Hopkins, J.A., and Sikela, J.M. 1991. Efficient double
stranded sequencing of cDNA clones containing long poly(A) tails using anchored
poly(dT) primers. Nucleic Acids Res. 19: 1715.
Landre, P.A., Gelfand, D.H., and Watson, R.M. 1995. The use of cosolvents to
enhance amplification by the polymerase chain reaction. In PCR Strategies, ed. Innis,
M.A., Gelfand, D.H., and Sninsky, J.J. Academic Press: San Diego, CA, pp. 3–16.
Lee, L.G., Spurgeon, S.L., Heiner, C.R., Benson, S.C., Rosenblum, B.B., Menchen,
S.M., Graham, R.J., Constantinescu, A., Upadhya, K.G., and Cassel, J.M. 1997. New
energy transfer dyes for DNA sequencing. Nucleic Acids Res. 25: 2816–2822.
Lobet, Y., Peacock, M.G., and Cieplak, W., Jr. 1989. Frame-shift mutation in the lacZ
gene of certain commercially available pUC18 plasmids. Nucleic Acids Res.
17: 4897.
Kieleczawa, J. 2005. DNA Sequencing: Optimizing the Process and Analysis.
Sudbury MA: Jones and Bartlett Publishers, Inc. 224 pp.
McMurray, A.A., Sulston, J.E., and Quail, M.A. 1998. Short-insert libraries as a
method of problem solving in genome sequencing. Genome Res. 8: 562–566.
Mills, D.R., and Kramer, F.R. 1979. Structure-independent nucleotide sequence
analysis. Proc. Natl. Acad. Sci (USA) 76: 2232–2235.
Mizusawa, S., Nishimura, S., and Seela, F. 1986. Improvement of the dideoxy chain
termination method of DNA sequencing by use of deoxy-7-deazaguanosine
triphosphate in place of dGTP. Nucleic Acids Res. 14: 1319–1324.
Molecular Probes, Inc. 1995. User Bulletin #MP-7581/4-01-95: Picogreen dsDNA
Quantitation Reagent (PN P-7581).
Rosenblum, B.B., Lee, L.G., Spurgeon, S.L., Khan, S.H., Menchen, S.M., Heiner,
C.R., and Chen, S.M. 1997. New dye-labeled terminators for improved DNA
sequencing patterns. Nucleic Acids Res. 25: 4500–4504.
Tabor, S., and Richardson, C.C. 1990. DNA sequence analysis with a modified
bacteriophage T7 DNA polymerase: effect of pyrophosphorolysis and metal ions. J.
Biol. Chem. 265: 8322–8328.

284
Bibliography
DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Tabor, S., and Richardson, C.C. 1995. A single residue in DNA polymerases of
Escherichia coli DNA polymerase I family is critical for distinguishing between
deoxy- and dideoxynucleotides. Proc. Natl. Acad. Sci (USA) 92: 6339–6343.
Thomas, M.G., Hesse, S.A., McKie, A.T., and Farzaneh, F. 1993. Sequencing of
cDNA using anchored oligo dT primers. Nucleic Acids Res. 16: 3915–3916.
Thweatt, R., Goldstein, S., and Shmookler Reis, R.J. 1990. A universal primer
mixture for sequence determination at the 3´ ends of cDNAs. Anal. Biochem.
190: 314–316.

285DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Glossary
ABI basecaller An algorithm used to determine the bases of a sequence. This algorithm is available
in Sequencing Analysis Software v5.x, SeqScape Software v2.x, and MicroSeq ID
Analysis Software. Development on this algorithm ended in 2003.
allele One member of a pair or series of alternative forms of a genetic locus.
analysis protocol The settings that govern sample analysis: basecalling, mixed base identification, clear
range and trimming, sequence file formats, and filtering.
assembly The aligned and overlapping sample data that result from the sequencing of one PCR
product or clone.
Assembly view In SeqScape software, a view of the specimen consensus sequence, the aligned sample
sequences, electropherograms, and quality values.
base spacing The number of data points from one peak to the next. A negative spacing value or a
spacing value shown in red indicates a problem with your samples, and/or the analysis
parameters.
basecaller An algorithm that determines the bases of a sequence during analysis.The two types
of basecallers are KB and ABI.
chromatogram See electropherogram.
clear range The region of sequence that remains after excluding the low-quality or error-prone
sequence at both the 5´ and 3´ ends. If the KB basecaller was used for analysis, the
clear range is calculated from the quality values (QVs). If an ABI basecaller was used,
the range is calculated from the Ns in the data and/or trim by the number of bases at
the start and end of the data.
complement The opposite strand of double-stranded DNA. For example, if you sequenced the 3´ to
5´ strand, then the 5´ to 3´ strand is the complement.
consensus
sequence
The output of the assembly from a biologically related group of samples.
data point A sampling of fluorescence. Each data point is associated with a scan number.
DyeSet/Primer file See mobility file.
electropherogram A multi-color picture of a sequence showing peaks that represent the bases.

286 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Glossary
ept A multi-color graph displaying the values for the voltage, power, current, and
temperature for the entire run.
FASTA format A standard text-based file format for storing one or more sequences.
heterozygote A position at which the electropherogram displays more than one base.
HIM Heterozygous insertion/deletion (indel) mutation.
IUB/IUPAC International Union of Biochemistry/International Union of Pure and Applied
Biochemistry.
KB basecaller An algorithm used to determine the bases of a sequence. The algorithm can calculate
mixed or pure bases and determines sample quality values. This algorithm is available
in Sequencing Analysis Software v5.x, SeqScape Software v2.x, MicroSeq ID
Analysis Software and Variant Reporter Software. Development on this algorithm is
ongoing.
layout view View of the layout of the sample assembly with arrows indicating the placement and
orientation of samples.
length The number of characters a sequence contains, including gap characters. For example,
GAATTC has a length of 6 and GAA-TTC has a length of 7.
length of read The usable range of high-quality or high-accuracy bases, as determined by quality
values. This information is displayed in the Analysis report.
mixed bases One-base positions that contain 2, 3, or 4 bases. These bases are assigned the
appropriate IUB code.
mobility file Files that compensate for the mobility differences between the dyes and primers and
correct the color-code changes due to the chemistry used to label the DNA. Mobility
files are sometimes referred to as DyeSet/Primer files.
noise Average background fluorescent intensity for each dye.
*.phd.1 file File format that can be generated during sample analysis. The file contains base calls
and quality values.
quality values An estimate (or prediction) of the likelihood that a given basecall is in error. Typically,
the quality value is scaled following the convention established by the phred program:
QV = -10 log10(Pe), where Pe stands for the estimated probability that the call is in
error. Quality values are a measure of the certainty of the basecalling and consensus-
calling algorithms. Higher values correspond to lower chance of algorithm error.
Sample quality values refer to the per-base quality values for a sample, and consensus
quality values are per-consensus quality values.
raw data A multi-color graph displaying the fluorescence intensity (signal) collected for each
of the four fluorescent dyes.

287DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Glossary
Reference Data
Group (RDG)
The data that contain the reference and associated data.
sample data The output of a single lane or capillary on a sequencing instrument. Sample data is
entered into Sequencing Analysis, SeqScape, and other sequencing analysis software.
sample files A file containing raw DNA sequence data (as read by the electrophoresis instrument),
and the basecalls, peak locations, and electropherogram created by the Sequencing
Analysis software. For Applied Biosystems genetic analysis instruments, raw sample
files are created and, optionally, analyzed by the Data Collection Software. Raw or
previously analyzed sample files are analyzed by Sequencing Analysis software.
Sample Manager A window that displays sample file name, name, and specimen; last used basecaller
and mobility (DyeSet/Primer) files; calculated basecalling results (spacing, peak 1,
start and stop); and assembly status. The sample name, basecaller, and/or mobility file
can be changed here.
sample score The average of the per-base quality values for the bases in the clear-range sequence
for the sample.
scan number On an Applied Biosystems genetic analysis instrument, one sampling taken during
each scan and stored as a data point.
*.scf file A file format that can be generated during sample analysis. The file contains base
calls, an electropherogram, and quality values but no raw data. Note: When standard
chromatogram file format is created, the .scf extension is not appended to the file
name. However, the file format is correct.
*.seq files Text files, created by the Sequencing Analysis software, containing only the
characters of the sequence. The *.seq files can be saved in ABI or FASTA format for
use with other software.
sequence A linear series of nucleotide base characters that represent a linear DNA sequence, or
a linear series of amino acid characters that represent a protein sequence, displayed in
rows from left to right.
sequencing
reactions
The reactions performed to incorporate fluorescent dye labels into DNA extension
products.
signal A number that indicates the intensity of the fluorescence from one of the dyes used to
identify bases during a data run. Signal strength numbers are shown in the Annotation
view of the sample file.
signal:noise The average of the signal intensity of the ‘A’, ‘C’, ‘G’, or ‘T’ base divided by the
average noise for that base.
spacing See base spacing.
specimen The container that holds all the sample data as assembled contigs from a biological
source or PCR product.

288 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
Glossary
specimen view In SeqScape software, a view of the consensus sequence and all sample files that were
used to create that consensus sequence.
variants Bases where the consensus sequence differs from the reference sequence that is
provided.

DNA Sequencing by Capillary Electrophoresis Chemistry Guide 289
Index
Symbols
*.phd.1 file
format 286
how to create 144
*.scf file
format 287
how to create 144
*.seq file
format 287
how to create 144
Numerics
310 instrument
part numbers for capillary consumables 272
part numbers for parts and documents 276
run modules 140
run parameters 155
3100 instrument
part numbers for capillary consumables 271
part numbers for parts and documents 275
run modules 139
run parameters 153
3100-Avant instrument
part numbers for capillary consumables 271
part numbers for parts and documents 274
run modules 139
run parameters 154
3130/3130xl instrument
part numbers for capillary consumables 270
part numbers for parts and documents 274
run modules 138
run parameters 152
3730/3730xl instrument
part numbers for capillary consumables 270
part numbers for parts and documents 273
run modules 138
run parameters 151
7-deaza-dGTP, use of in BigDye
®
primer kits 60
A
ABI basecaller
compared to KB
™
basecaller 144
defined 285
absorbance
using to examine DNA quality 44
using to quantitate DNA 45
agarose gel electrophoresis, to examine DNA
quality 44
allele, defined 285
analysis protocol
basecalling settings 144
clear range settings 150
creation of in electrophoresis workflow 129
defined 285
defining settings 143
general settings 143
mixed bases settings 149
analysis. See data analysis.
annotation tab, reviewing 197
Applied Biosystems
contacting viii
Technical Support viii
artifacts, reviewing for 196
assembly view, defined 285
assembly, defined 285
assumptions for using this guide vii
AT-rich sequences, recommended kits 56
autoanalysis
overview 12
average signal intensity
high values 206, 214, 225, 239, 242, 252
low value for C 231
reviewing 197
B
bacterial artificial chromosome (BAC) DNA template
commercial products for preparing 34
preparing 33
quantity recommended 63
recommended sequencing kits for 56
bacterial genomic DNA template
quantity recommended 63
recommended sequencing kits for 56
base complements 3
base spacing
defined 285
reviewing 197
basecaller
appropriate location for file 195
defined 285
selecting 144
verifying correct one is used 195

290 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
basecalling
mixed bases not called correctly 204
overview 11
settings for 144
stops 219
too many mixed bases called 205
baseline
negative 216, 217
reviewing 196
waterfall symptom 218
BDX Update utility 210
Beer’s Law
derivation of to calculate primer concentration 39
using to calculate DNA template quantity 45
BigDye
®
primers 73
application recommendations 56
chemistry overview 7
description 55
DNA template quantity recommended 63
electropherogram example 60
kit advantages 60
part numbers 268
peak color/base relationships 262
pGEM-3Zf(+) control sequence 263
purifying extension products 114
reactions using 73
BigDye
®
terminators 66
advantages 57
application recommendations 56
chemistry overview 6
description 55
direct sequencing from cultures 75
DNA template quantity recommended 63
dye set 133
electropherogram example 57
irregular C peaks symptom 231
irregular G peaks symptom 233
kit part numbers 266
M13 control primer sequences 263
part numbers 266, 267
peak color/base relationships 262
pGEM-3Zf(+) control sequence 263
purifying extension products 96
reactions using 66
sequencing standard sequence 264
spectral calibration standard for 133
BigDye
®
XTerminator
™
Purfication Kit 88
about 88
BDX Update utility 210
part numbers 266
premix pipetting protocol 91
run modules 140
sequential pipetting protocol 93
symptom of incorrect run module 225
bisulfite conversion 23
genomic DNA template, recommended quantity 63
bisulfite sequencing
basecaller 144
broad peaks 236
double peaks after homopolymer 247
double peaks at the beginning 241
peaks under peaks throughout 244
thermal cycling conditions 79
blobs in electropherogram 230
bold text, when to use vii
buffer recommendations 130
C
C peaks, irregular 231
calibrating the instrument 131
capillary electrophoresis
See also electrophoresis.
array lengths supported 10
capillaries 130
capillaries per instrument 10
consumable part numbers 270
instrument consumables 129
overview 9, 128
preparing samples for 122
using Applied Biosystems instruments 10
CAUTION, description x
chemical safety xi
chromatogram. See electropherogram.
clear range
defined 285
settings for 150
color/base relationships 262
comparative sequencing, recommended kits for 56
complement, defined 285
compressions, of peaks 235
condition number 134
consensus sequence, defined 285
controls
DNA template controls 64
M13 control primer sequences 263
pGEM-3Zf(+) sequence 263
sequencing standard part numbers 268
sequencing standard sequence 264
sequencing standards 129
troubleshooting using 194
conventions
bold text vii
for describing menu commands vii
IMPORTANTS! vii
in this guide vii
italic text vii
Notes vii
safety x
user attention words vii
cosmid DNA
quantity recommended 63

DNA Sequencing by Capillary Electrophoresis Chemistry Guide 291
recommended sequencing kits for 56
cultures, direct sequencing from 75
current during run, reviewing 198
cycle sequencing
Applied Biosystems kits 7
choosing chemistry for 55
DNA quantities recommended 63
handling sequencing reagents 61
kit descriptions 55
kit recommendations 56
overview 5
peak color/base relationships 262
storing reactions 61
using BigDye
®
primers 73
using BigDye
®
terminators 66
using dGTP BigDye
®
terminators 66
using DNA template controls 64
using dRhodamine terminators 72
D
DANGER, description x
data analysis
defining analysis protocols for 143
overview 11
PCR stop point 219
reviewing information 196
reviewing settings 195
settings on annotation tab 197
using MicroSeq
®
ID Analysis Software 188
using SeqScape
®
Software 188
using Sequence Scanner Software 164
using Sequencing Analysis Software 170
when mobility file is incorrect 229
with incorrect mixed bases settings 204
with incorrect quality threshold setting 204
Data Collection Software
in electrophoresis workflow 129
settings 136
settings on Annotation tab 197
data start point, reviewing 196
de novo sequencing, recommended kits for 56
dGTP BigDye
®
terminators
advantages 58
application recommendations 56
chemistry overview 6
description 55
DNA template quantity recommended 63
dye set 133
electropherogram example 58
kit part numbers 267
M13 control primer sequences 263
peak color/base relationships 262
peak compression symptom 235
pGEM-3Zf(+) control sequence 263
purifying extension products 105
reactions using 66
spectral calibration standard for 133
dideoxy sequencing 4
direct sequencing from cultures 75
DNA polymerase
in cycle sequencing 8
in DNA synthesis and replication 2
in Sanger dideoxy sequencing 4
DNA quality
determining 44
troubleshooting 199
DNA quantity
determining 45
recommendations for cycle sequencing 63
troubleshooting 199
DNA template
BAC DNA, preparing 33
cleaning dirty templates 44
commercial products for preparing 34, 36, 41
contaminants in 44
controls for 64
determining quality of 44
difficult to sequence 220
genomic DNA, preparing 34
PCR DNA, preparing 37
plasmid DNA, preparing 33
quantitation of 45
quantities recommended for cycle sequencing 63
single-stranded DNA, preparing 33
vector-based DNA, preparing 33
double peaks
after homopolymer or repeated sequence 246, 247
double sequence after clean sequence 248
n+1 or n-1 sequence throughout 244
peaks under peaks throughout 237
specific peaks under specific bases 242, 243
with h
ig
h average signal intensity values 239
double-stranded DNA
quantitating 45
quantity recommended 63
dRhodamine terminators
advantages 59
application recommendations 56
chemistry overview 6
description 55
DNA template quantity recommended 63
dye set 133
electropherogram example 59
kit part numbers 267
M13 control primer sequences 263
part numbers 267
peak color/base relationships 262
pGEM-3Zf(+) control sequence 263
purifying extension products 109
reactions using 72
sequencing standard sequence 264
spectral calibration standard for 133
dye degradation

292 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
preventing 61, 122
symptom of 231, 233, 234
dye primers
emission spectra of dyes 8
features of chemistry 7
dye set, correct set for cycle sequencing kits 133
dye terminators
emission spectra of dyes 8
features of chemistry 6
DyeSet/Primer files. See mobility files.
E
editing bases
Sequence Scanner Software 164
Variant Reporter
™
Software 177
electrokinetic injection, modifying 141
electropherogram
defined 285
example 11, 195
example using BigDye
®
primers 60
example using BigDye
®
terminators 57
example using dGTP BigDye
®
terminators 58
example using dRhodamine terminators 59
peak color/base relationships in 262
reviewing 195
electrophoresis
description 4
modifying conditions for 142
optimizing conditions for 142
preparing samples for 122
procedural overview 129
run temperature and voltage 143
run time 143
epigenetics See methlyation.
EPT tab, reviewing 198
ethanol precipitation, to purify extension products 96
examples
electropherogram 195
EPT tab 198
raw data 196
troubleshooting symptoms 201
ExoSAP-IT, using for purifying PCR reactions 43
expressed sequence tags 20
extension product purification
alternative procedures 119
BigDye XTerminator Purification Kit 88
ethanol precipitation 96
spin column and spin plate purification 116
extension products
about creation of 2
how labeled 6
F
failure, of capillaries 130
FASTA format, defined 286
file format
.phd.1 286
.scf 287
.seq 287
FASTA 286
formamide, resuspending samples in 122
formulas
Beer’s Law 45
DNA quantity using A
260
45
melting temperature 39
oligonucleotide molecular weight 39
primer concentration 39
fosmid DNA
recommended sequencing kits for 56
G
G peaks, irregular 233
gap closure (custom primers), recommended sequencing
kits for 56
GC-rich sequences, recommended sequencing kits
for 56
gene walking (custom primers), recommended
sequencing kits for 56
general settings, for analysis 143
genomic DNA
commercial products for preparing 36
preparing 34
genomic DNA, bacterial
quantity recommended 63
recommended sequencing kits for 56
GT-rich regions, recommended sequencing kits for 56
H
hazard symbols. See safety symbols, on instruments
heat seal, using 122
heparin, in genomic DNA 35
heterozygotes, verifying 180, 188
heterozygous indel mutation (HIM) detection 248
Hi-Di™ Formamide, using 122
homopolymeric regions
recommended sequencing kits for 56
troubleshooting sequencing of 206, 234, 238, 246,
247
I
IMPORTANT, description x
indel, detection of 248
injection
modifying 141
overview 9
instrument

DNA Sequencing by Capillary Electrophoresis Chemistry Guide 293
calibration of 131
part numbers 270
preparation of electrophoresis workflow 129
instrument protocol
creation of in electrophoresis workflow 129
list of settings 137
instrument run
determining parameters for 151
determining required run time 143
modifying conditions 142
reviewing 196
selecting a run module 137
italic text, when to use vii
IUB/IUPAC
defined 286
website 282
K
KB basecaller
compared to ABI basecaller 144
defined 286
in Phred software workflow 11
Ns in sequence 171
L
lambda DNA, recommended sequencing kits for 56
layout view, defined 286
literature references 281 to 282
M
M13 control primers
sequence of 263
use in sequencing 22
M13mp18 control
troubleshooting using 194
using 64
magnetic bead adsorption/elution products for extension
product purification 119
manual analysis
overview 12
matrix file
appropriate location for 195
verifying correct one used 195
matrix standards
about 132
part numbers 268
menu commands, conventions for describing vii
methylation 23
microbial analysis 27
MicroSeq
®
ID Analysis Software
product part numbers 278
reviewing results 188
workflow 189
miniprep kits 34
mixed bases
defined 286
defining settings for 149
not called correctly 204
too many called 205
MLST
about 27
URL for website 28
mobility file
appropriate location for 195
defined 286
verifying correct one used 195
mobility shift correction, description 11
molecular weight, calculating for DNA
oligonucleotide 39
mRNA 20
MSDSs
description xi
obtaining viii
Multi Locus Sequence Typing See MLST.
multicomponent analysis, description 11
mutation detection 188
N
n+1 or n-1 sequence throughout 244
nested PCR 37
noise, defined 286
O
overviews
Applied Biosystems automated DNA sequencing 5
capillary electrophoresis 9
cycle sequencing 5
data analysis 10, 158
DNA synthesis and replication 2
dye primer cycle sequencing 7
dye terminator cycle sequencing 6
electrophoresis 4
sanger dideoxy sequencing 4
P
paraffin-embedded tissue, considerations for PCR 35
PCR amplicons
recommended sequencing kits for 56
PCR DNA template
commercial products for preparing 41
contaminants in 40
preparing 37
purifying for sequencing 41
quantity recommended 63
PCR stop point 219
peak heights, reviewing in raw data 196

294 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
peak symptoms
blobs 230
compressed peaks 235
double peaks 239, 240, 242, 243, 244, 246, 248
irregular C peaks 231
irregular G peaks 233
large peaks 230
missing C peaks 231
n+1 or n-1 sequence throughout 244
peaks under peaks throughout 237
resolution loss 250, 251
shoulders 231, 234
spikes 226, 227
split peaks 225
unevenly spaced peaks 229
pGEM-3Zf(+) control
sequence 263
troubleshooting using 194
using 64
Phred, creating Phred (.phd.1) file 144
plasmid DNA template
commercial products for preparing 34
preparing 33
recommended sequencing kits for 57
plate linking, in electrophoresis workflow 129
plate record
creation of in electrophoresis workflow 129
list of settings 136
plates, part numbers for 269
poly(A) regions, sequencing 247
poly(T) regions, sequencing 247
polymer
problems caused by crystals 226
problems caused by heating 226, 227
recommendations 130
supported types 10
power during run, reviewing 198
primer walking 18
primers
designing 38, 61
ordering custom 39
primer-dimer formation 224
quantitation of 38, 61
re
view
ing design of 200
software for designing 278
protocol part numbers 269
purifying extension products
ethanol precipitation 96
overview 86
using BigDye XTerminator Purification Kit 88
using magnetic bead adsorption/elution 119
using size exclusion products 119
using spin columns or spin plates 116
Q
Q Value 134
quality values (QVs)
chart 172
defined 286
overview 11
quantitation
of DNA template 45
of primers 38, 61
R
raw data
defined 286
example of high quality 196
in calibration results 134
peak color/base relationships in 262
reviewing 196
reaction plates, part numbers 269
read length
for 310 instrument run modules 140
for 3100 instrument run modules 139
for 3100-Avant instrument run modules 139
for 3130/3130xl instrument run modules 138
for 3730/3730xl instrument run modules 138
read length, reviewing 196
Ready Reaction Mix
blended 234, 252
improper storage 208
insufficient quantity 207, 211, 220, 221, 222, 223
Reference Data Group (RDG)
creating 184
definition 287
references, literature 281 to 282
reptation peak 228
resequencing 20
resolution
improving 142
loss of 214, 250, 251
results group, creation of in electrophoresis
workflow 129
resuspending purified sequencing products 122
RNA, using as starting material 35
rolling circle amplified product
recommended sequencing kits for 57
run module
modifying 141
selecting 137
run. See instrument run.
S
safety
biological hazards xi
chemical xi

DNA Sequencing by Capillary Electrophoresis Chemistry Guide 295
conventions x
sample
capacity supported on Applied Biosystems
instruments 10
data, defined 287
loading in electrophoresis workflow 129
preparing for electrophoresis 122
Sample Manager
defined 287
example 195
red spacing value in 203
sample score, defined 287
Sanger dideoxy sequencing 4
SAP/Exo I, using to purify PCR reactions 42
SDS/heat treatment, in spin column and spin plate
purification 116
semi-nested PCR 37
SeqScape
®
Software
detection of HIM using 248
high quality sequence in unassembled category
symptom 253
product part numbers 278
workflow 181
sequence
M13 control primers 263
pGEM-3Zf(+) control 263
sequence file format, setting 144
Sequence Scanner Software
editing bases 164
workflow 164
sequencing
potential contaminants in 44
Sanger dideoxy sequencing 4
storing reactions 121
Sequencing Analysis Software
gray quality value bars 171
product part numbers 278
quality values chart 172
red spacing value in 203
reviewing data analysis settings 195
reviewing run and analysis information 196
workflow 170
sequencing primers
nested 22
See also primers.
tailed 22
sequencing standards
about 132
part numbers 268
troubleshooting using 129, 194
shotgun sequencing
about 18
recommended sequencing kits for 56
shoulders
on all peaks 234
on C peaks 231
shrimp alkaline phosphatase (SAP) digestion, using to
purify extension products 110
signal symptoms
excessive signal 225
low signal 207, 209, 211
negative baseline 216, 217
no signal 207, 209
signal starts later than expected 213, 214
ski slope profile 223
spike 226, 227, 228
sudden drop in signal 21
9,
220, 221
top-heavy data 222, 223, 224, 225
waterfall baseline 218
signal/noise
defined 287
reviewing 197
single-stranded DNA template
commercial products for preparing 34
preparing 33
quantitating 45
quantity recommended 63
recommended sequencing kits for 57
size exclusion products, for extension product
purification 119
ski slope profile 223
spacing of peaks, troubleshooting 229
spacing value, red 203
spatial calibration
about 131
performing in electrophoresis workflow 129
specimen, defined 287
spectral calibration
about 131
log file 135
performing in electrophoresis workflow 129
spectral profile 134
spectral pull-up 237
spectrophotometry, using to examine DNA quality 44
spikes
at end of run 228
four-color 226
one-color 227
spin column and spin plate purification 116
split peaks 225
standards
for sequencing 129
for spectral calibration 132
stutter 206, 234, 238, 246, 247
symptoms
blobs in electropherogram 230
double peaks 237, 239, 240, 242, 243, 244, 24
6,
248
ex
cessive signal 225
high quality sequence in unassembled category 253

296 DNA Sequencing by Capillary Electrophoresis Chemistry Guide
irregular C peaks 231
irregular G peaks 233
large peaks 230
low signal 207, 209, 211
missing C peaks 231
mixed bases not called correctly 204
n+1 or n-1 sequence throughout 245
negative baseline 216, 217
no signal 207, 209
peak compressions 235
peak shoulders 234
peaks under specific bases 242, 243
peaks unevenly spaced 229
resolution loss 214, 250, 251
shoulders on peaks 231
signal drops suddenly 219, 220, 221
signal starts later than expected 213, 214
ski slope profile 223
spikes 226, 227, 228
split peaks 225
table of 201
too many mixed bases called 205
top-heavy data 222, 223, 224, 225
waterfall baseline 218
T
Technical Support, contacting viii
temperature of run
modifying 143
reviewing 198
template
BAC DNA, preparing 33
contaminants in 44
genomic DNA, preparing 34
PCR DNA, preparing 37
PCR DNA, purifying 41
plasmid DNA, preparing 33
single-stranded DNA, preparing 33
vector-based DNA, preparing 33
text conventions vii
thermal cyclers
recommended 62
selecting 62
thermal cycling conditions
bisulfite-converted DNA 79
DNA template controls 65
using BigDye
®
primers 74
using BigDye
®
terminators 67
using dRhodamine terminators 72
top-heavy data 222, 223, 224, 225
training, information on viii
troubleshooting
DNA quantity and quality, reviewing 199
EPT tab 198
index 255
overview 194
primer design, reviewing 200
symptoms table 201
workflow 194
tube septa, using 122
tubes
part numbers 269
selecting for thermal cycler 62
U
universal-tailed PCR primers 37
user attention words, described vii
UV absorbance, for DNA quantitation 45
V
Variant Reporter
™
Software
editing bases 177
product part numbers 278
project data folder 178
workflow 175
variants, defined 288
vector-based DNA template
commercial products for preparing 34
preparing 33
voltage
modifying 143
reviewing 198
W
WARNING, description x
waterfall baseline 218
workflows
automated DNA sequencing 13
BigDye
®
XTerminator™ Purification Kit 89
bisulfite sequencing 26
capillary electrophoresis 129
KB basecaller and Phred software 11
MicroSeq
®
ID Analysis Software 189
MLST (Multi Locus Sequence Typing) 27
SeqScape
®
Software 181
Sequence Scanner Software 164
Sequencing Analysis Software 170
troubleshooting 194
Variant Reporter
™
Software 175

Part Number 4305080 Rev. C 05/2009
Technical Resources and Support
For the latest technical resources and support information
for all locations, please refer to our Web site at
www.appliedbiosystems.com/support
Applied Biosystems
850 Lincoln Centre Drive | Foster City, CA 94404 USA
Phone 650.638.5800 | Toll Free 800.345.5224
www.appliedbiosystems.com

