
Clinical Procedure Safety
Summary
This Policy Directive addresses clinical care and patient safety risks associated with
clinical procedures; improves matching of the patient to the correct procedure;
improves communication within the procedural team, and between the patient and the
procedural team; and reduces the number of clinical procedure related incidents.
Document type
Policy Directive
Document number
PD2017_032
Publication date
22 September 2017
Author branch
Clinical Excellence Commission
Branch contact
(02) 9269 5500
Review date
02 September 2024
Policy manual
Not applicable
File number
CEC 17/238
Status
Review
Functional group
Clinical/Patient Services - Governance and Service Delivery
Applies to
Affiliated Health Organisations, Dental Schools and Clinics, Local Health Districts,
NSW Health Pathology, Public Hospitals, Specialty Network Governed Statutory
Health Corporations
Distributed to
Government Medical Officers, Ministry of Health, NSW Ambulance Service, Private
Hospitals and Day Procedure Centres, Public Health System, Tertiary Education
Institutes
Audience
All clinical staff
Policy Directive
Secretary, NSW Health
This Policy Directive may be varied, withdrawn or replaced at any time. Compliance with this directive is
mandatory for NSW Health and is a condition of subsidy for public health organisations.

POLICY STATEMENT
PD2017_032
Issue date: September-2017
Page 1 of 1
CLINICAL PROCEDURE SAFETY
PURPOSE
The purpose of this policy directive is to address clinical care and patient safety risks
associated with clinical procedures; improve matching of the patient to the correct
procedure; improve communication within the procedural team, and between the patient
and the procedural team; and reduce the number of clinical procedure related incidents.
The principles of the World Health Organization (WHO) Surgical Safety Checklist and
the Royal Australasian College of Surgeons’ Surgical Safety Checklist have been used
in the development of this policy directive.
This policy directive aligns with the National Safety and Quality Health Services
Standards requirements for correctly matching patients with their intended care.
MANDATORY REQUIREMENTS
All staff involved in clinical procedures must adhere to the requirements of this policy
directive regardless of the location where the procedure is performed.
Each health service undertaking clinical procedures must have systems and processes
in place to enable compliance with this policy directive. This includes educating and
training staff, documenting incidents associated with procedures, monitoring compliance
with this policy directive, and reporting outcomes to the appropriate committee/s within
the health service and to relevant external agencies such as the NSW Coroner’s office.
IMPLEMENTATION
Chief Executives are responsible for:
Assigning responsibility for implementing and complying with this policy directive
and reporting on the implementation of this policy document as required.
Clinicians are responsible for:
Complying with this policy directive.
Clinical Excellence Commission is responsible for:
Reviewing and ensuring the currency of this policy directive.
REVISION HISTORY
Version
Approved by
Amendment notes
PD2017_032
September 2017
Deputy Secretary, People, Culture
and Governance
Revised following review. Replaces
PD2014_036.
(PD2014_036)
October 2014
Deputy Secretary, Governance,
Workforce & Corporate
Revised following review. Replaces
PD2007_079.
PD2007_079
Director General
Revised following review. Replaces
PD2005_380
PD2005_380
Director General
New policy
ATTACHMENT
1. Clinical Procedure Safety: Procedures

Clinical Procedure Safety
PROCEDURES
Issue date: September-2017
PD2017_032

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Contents page
CONTENTS
1 BACKGROUND ........................................................................................................................ 1
1.1 About this document ......................................................................................................... 1
1.2 Principles ........................................................................................................................... 1
1.3 Key definitions ................................................................................................................... 2
2 LEVEL 1 PROCEDURES ......................................................................................................... 6
2.1 Pre procedure ................................................................................................................... 6
2.2 Post procedure .................................................................................................................. 8
3 LEVEL 2 PROCEDURES ......................................................................................................... 9
3.1 Pre procedure (including Team Time Out) ....................................................................... 9
3.2 Post procedure ................................................................................................................ 12
4 LEVEL 3 PROCEDURES ....................................................................................................... 14
4.1 Pre procedure requirements ........................................................................................... 15
4.2 Requirements for a Level 3 procedure checklist ............................................................ 17
4.3 Sign In One: Checklist completed by the sedationist / anaesthetist .............................. 19
4.4 Sign In Two: Checklist completed by the proceduralist ................................................. 22
4.5 Team Time Out – Checklist led by the senior proceduralist .......................................... 23
4.6 Sign Out – Checklist completed by the nurse / midwife ................................................. 25
5 INCIDENTS ............................................................................................................................. 27
6 AUDITING AND REPORTING ............................................................................................... 27
7 RESOURCES ......................................................................................................................... 28
8 ABBREVIATIONS .................................................................................................................. 28
9 REFERENCES ........................................................................................................................ 29
10 FURTHER READING ............................................................................................................. 31

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 1 of 31
1 BACKGROUND
1.1 About this document
The purpose of this policy directive is to address clinical care and patient safety risks
associated with clinical procedures; improve matching of the patient to the correct
procedure; improve communication within the procedural team and between the patient
and the procedural team; and reduce the number of clinical procedure related incidents.
1.2 Principles
The following principles apply to clinical procedures.
1. The policy directive applies to the full age range of patients. Where issues are
specific to children these are raised by way of exception for children.
2. The manager / departmental head is responsible for ensuring the processes for
clinical procedure safety are followed.
3. Every clinician involved in a procedure whether as an individual proceduralist or as
a member of a procedural team is responsible for ensuring the processes for
clinical procedure safety are followed.
4. Active involvement and effective communication between the proceduralist (and
procedural team members where appropriate) and the patient or their person
responsible should occur.
5. Use age appropriate communication techniques when communicating with
children. A staff member experienced in communicating with children should
provide an explanation of the procedure, in consultation with the person
responsible, in language that can be understood by the child. The use of toys such
as dolls or teddy bears may assist with explanations as may the opportunity to see
and touch any non-dangerous equipment prior to the procedure such as a
stethoscope and the anaesthetic mask.
6. In general, for Level 1 and Level 2 procedures, the person responsible is
encouraged to stay with their child where clinically appropriate and where the child
is conscious, and agreed between the senior proceduralist and the person
responsible; for Level 3 procedures up to when the child is sedated / anesthetised
and then following the procedure as the child wakes up as the clinical situation
allows.
7. Valid consent must be obtained for the procedure.
1
8. The proceduralist (and procedural team members where appropriate) is
responsible for confirming patient identification, procedure verification and where
appropriate the correct site / side / level for the procedure. The proceduralist
carries ultimate responsibility for the patient identification and procedure
verification.
9. Patient identification, and verification of the correct procedure and correct site
(where appropriate) must occur prior to the procedure commencing.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 2 of 31
10. To the extent possible involve the patient, or their person responsible, at all points
in the patient identification and procedure verification processes, including marking
of the procedure site, where appropriate.
11. Site marking is essential where there is the potential for error involving multiple
structures (fingers, toes, or lesions), left / right distinction, or levels (spine).
12. Confirm the patient’s known allergies / adverse reactions to substances. Ensure
substances the patient has a known allergy / adverse reaction to are not used
during the procedure.
13. If pre-procedure imaging data are to be used, the data must be available and
correctly identified before the patient receives procedural sedation / anaesthesia.
14. If prostheses, implants, sterile equipment, or special equipment are required, they
must be available and, where appropriate, confirmed they are functional and
appropriate for use e.g. left / right, before the patient receives procedural sedation /
anaesthesia.
1.3 Key definitions
Airway management
Includes oxygen therapy via face mask, management of airways
obstruction including the use of common devices such as oro -
pharyngeal and naso - pharyngeal airways, single handed and two
handed mask ventilation using Bag and Mask, insertion and
management of Laryngeal Mask Airways and intubation of the
trachea using standard laryngoscopy equipment and monitoring of
the patient for the effects of hypoxia with basic monitoring such as
ECG (electrocardiogram), NIBP (non-invasive measurement of
blood pressure), Pulse Oximetry and CO
2
waveform analysis for
deep sedation.
Anaesthesia and sedation
Refer to definition - Sedation and anaesthesia.
Assisting clinicians
Staff engaged in assisting the proceduralist as part of the
procedure.
Clinical handover
The effective transfer of professional responsibility and
accountability for some or all aspects of care for a patient, or
group of patients, to another person or professional group on a
temporary or permanent basis.
2
Clinician
A person authorised by a facility to provide clinical care to a
patient.
Clinician airway monitor
A dedicated clinician (who is not the proceduralist) with
appropriate competency-based training, whose primary
responsibility is to monitor the patient’s level of consciousness
and to monitor and provide the initial management of cardio-
respiratory status of the patient during the procedure.
Incident
Any unplanned event resulting in, or with the potential for, injury,
damage or other loss. This includes a near miss.
3
Must
Indicates a mandatory action required that must be complied with.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 3 of 31
Patient
A person receiving health care. Also known as consumer or client.
Patient identification
The active process of confirming a patient’s identity through the
use of approved patient identifiers to ensure the correct patient is
matched to their planned procedure.
4
Person responsible
For the purposes of this policy directive a person responsible is a
person who can provide consent for a patient’s clinical procedure
to be performed.
1
Proceduralist
A clinician who is performing or assisting in the procedure.
There may be more than one proceduralist involved in a
procedure. The senior proceduralist takes overall responsibility
for the case.
Procedural Team
Includes all clinicians participating in the delivery of care
during the procedure.
Procedure
For the purposes of interpreting this policy directive procedure
is defined as follows.
Level 1 procedure
•
Usually requires a single proceduralist
•
Usually does not require written consent
•
Does not involve procedural sedation or general
/ regional anaesthesia.
Exception - Dental procedures involving dental
nerve blocks are classified as Level 1
procedures.
•
Usually performed in wards, emergency
departments, clinics and imaging departments.
Level 2 procedure
•
Requires a proceduralist, often supported by an
assisting proceduralist/s
•
Usually requires written consent
•
Does not involve procedural sedation or general
/ regional anaesthesia
•
Usually performed in wards, emergency
departments, clinics, imaging departments and
interventional suites.
Level 3 procedure
•
Requires at least one proceduralist and a procedural
team
• Always requires written consent
• Involves procedural sedation or general / regional
anaesthesia

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 4 of 31
• Usually performed in formal procedural suites such as
operating theatres, emergency departments,
endoscopy suites, interventional imaging suites,
birthing suites and cardiac catheterisation laboratories.
Procedure verification
The active process of verifying the procedure by confirming the
planned procedure and the site / side / level for the procedure.
Sedation and anaesthesia
5
Procedural sedation implies that the patient is in a state of
drug-induced tolerance of uncomfortable or painful diagnostic or
interventional medical, dental or surgical procedures.
•
Conscious sedation is defined as a drug-induced
depression of consciousness during which patients are
able to respond purposefully to verbal commands or
light tactile stimulation.
•
Deep levels of sedation, where consciousness is lost
and patients only respond to painful stimulation, are
associated with potential loss of the ability to maintain a
patent airway, inadequate spontaneous ventilation and /
or impaired cardiovascular function. Deep levels of
sedation may have similar risks to general anaesthesia,
and may require an equivalent level of care.
For the purposes of interpreting this policy directive:
•
Use of opioids
The use of opioids for analgesia is not considered procedural
sedation.
•
Use of nitrous oxide
- If the primary intent is analgesia then it is not considered
procedural sedation.
- If the primary intent is sedation then it is considered
procedural sedation and these procedures must be
classed as Level 3 procedures.
Procedural sedation does NOT include premedication to
reduce anxiety or provide pain relief.
Regional anaesthesia includes major nerve blocks, epidural
blocks and spinal blocks. Excludes dental nerve blocks. It
involves the injection of local anaesthetic in the vicinity of major
nerve bundles supplying body areas. Regional anaesthesia may
be used on its own or combined with sedation or general
anaesthesia.
General anaesthesia is a drug-induced state characterised by
absence of purposeful response to any stimulus, loss of protective

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 5 of 31
airway reflexes, depression of respiration and disturbance of
circulatory reflexes. General anaesthesia is sometimes indicated
during diagnostic or interventional medical or surgical procedures
and requires the exclusive attention of an anaesthetist, or other
appropriately trained and credentialed medical specialist within
their scope of practice.
Should
Indicates a recommended action that should be followed unless
there are sound reasons for taking a different course of action.
Sign In
The period immediately before preparing the patient
for their procedure by the procedural team.
Sign Out
The period after the procedure and before the patient /
procedural team leaves the procedural area.
Team Time Out
The period immediately before commencing the procedure to
undertake a final verification of the patient’s identity and the
procedure. Team Time Out applies to Level 2 and Level 3
procedures.
VTE prophylaxis
Treatment, either pharmacological or mechanical, provided to a
patient in order to reduce the risk of venous thromboembolism
(deep vein thrombosis and pulmonary embolism).
6
Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 6 of 31
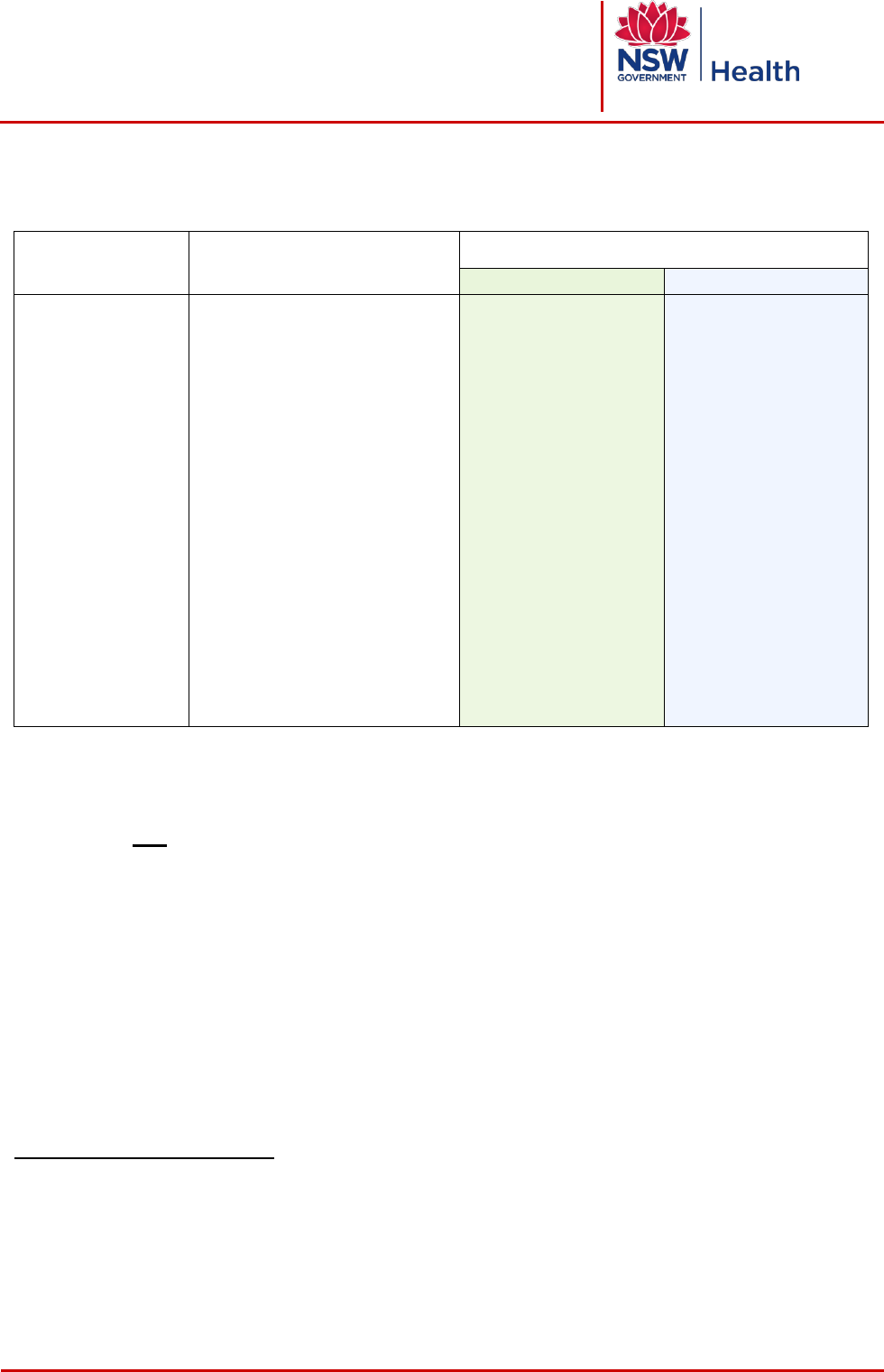
2 LEVEL 1 PROCEDURES
Definition
Examples
a
Requirements
Pre-procedure
Post procedure
- Single
proceduralist
- Usually does not
require written
consent
- Does not involve
procedural
sedation or
general/regional
anaesthesia,
except for dental
procedures
involving dental
nerve blocks
- Usually performed
in wards,
emergency
departments,
clinics, imaging
departments
- Insertion IV cannula
- Insertion IDC
- Insertion NGT
- Taking blood samples
- Diagnostic Radiology
- Diagnostic Nuclear Medicine
- Routine dental procedures e.g.
dental extraction, fillings
- Dental procedures involving
dental nerve blocks
- Superficial skin
lesions/biopsies
- Non operative obstetrics e.g.
fetal scalp blood sampling,
perineal repair with LA,
Artificial Rupture of
Membranes, fetal scalp
electrode
b
STOP and confirm the
following before
commencing the
procedure
- Patient identification
- Procedure verification
- procedure +
site/side/level, where
appropriate, matches
consent
- Allergy/adverse
reaction check
- Anticipated critical
events
- Document procedure
in patient’s health care
record or Radiology
Information System
- Advice for clinical
handover
- Label
specimen/images
- Post procedure tests
where clinically
relevant
2.1 Pre procedure
Procedures not involving procedural sedation / anaesthesia are either Level 1 or Level
2 procedures. Refer to the definition and examples for guidance in classifying
procedures as Level 1 or Level 2.
For Level 1 procedures the proceduralist, and assisting proceduralist/s, where
relevant, must STOP and confirm the following minimum requirements immediately
before commencing the procedure. Where two or more staff members are involved
they must introduce themselves to each other and the patient, as appropriate, by their
preferred names and roles before the procedure commences.
2.1.1 Patient identification
The patient’s identity must be confirmed before any procedure commences.
a
The examples provided do not cover all possible procedures and the examples may be escalated to a higher level (ie Level 1
procedures may be classified by a health service as Level 2 or Level 3 procedures). Health services should consider development
of local lists of examples for Level 1, Level 2 and Level 3 procedures consistent with the requirements of this policy directive.
b
Where the procedure is a non operative obstetric procedure and patient identification has occurred at the commencement of
labour, the obstetric team that has cared for the patient during labour should confirm the patient's identification immediately before
commencing the procedure if appropriate e.g. if the patient is moved to a new room or a new member joins the obstetric team
caring for the patient during the procedure.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 7 of 31
Staff must confirm that they have the correct patient by asking the patient, or their
person responsible, to state the patient’s full name and date of birth. Staff should
not state the patient’s name or date of birth and then ask the patient, or their
person responsible, if this information is correct.
The response must be confirmed against the details on the request form / referral /
treatment plan and patient identification band or other approved patient
identification tool (including unique patient identifier), as appropriate.
Where patient details on the request form / referral / treatment plan are incomplete
or there is a discrepancy with the information received from the patient, or their
person responsible, the correct information must be verified before commencing
the procedure and actions taken documented in the patient’s health care record.
If the patient is unable to participate in the patient identification step, for example
due to physical incapacity, language issues, or is a child, and their person
responsible is not present, then the patient’s identification band or other approved
patient identification tool (including unique patient identifier) should be used to
confirm the patient’s identification.
2.1.2 Procedure verification
Consent must be obtained for any procedure as required by the NSW Health policy
directive on consent to medical treatment.
1
Consent must be documented for high risk radiology and nuclear medicine
procedures for Diagnostic Imaging Accreditation Scheme accreditation.
Signed consent forms are not required for minor procedures performed under local
anaesthesia, e.g. insertion of IV cannula, urethral catheterisation, or suture of minor
lacerations.
Request forms / referrals / treatment plans for procedures must include the
patient’s name, date of birth, sex, unique patient identifier (where appropriate),
reason for the procedure, details of the test/s required, the date the test/s were
ordered, and the exact anatomical location for the test/s including the procedure
site, laterality and level.
The proceduralist must ask the patient, or their person responsible, to state what
procedure they understand will be performed and to state the site / side / level for
the procedure (where relevant) and verify this matches the planned procedure and
consent / request form / referral / treatment plan.
7
Where procedure details on the request form / referral / treatment plan are
incomplete or there is a discrepancy the requesting clinician or a member of their
team must be contacted to clarify the information before commencing the
procedure and the response documented.
2.1.3 Allergy / adverse reaction check
Ask the patient, or their person responsible, if they have a known allergy / adverse
reaction and if yes, what the allergy / adverse reaction was and what effect they
experienced. The response should be documented.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 8 of 31
2.1.4 Anticipated critical events
The proceduralist must consider the planned procedure, critical steps, anticipated
events and equipment requirements.
2.2 Post procedure
The name of the proceduralist/s must be documented in the patient’s health care
record or Radiology Information System.
Document the name of the procedure and outcome/s in the patient’s health care
record or Radiology Information System.
Provide clinical handover advice (verbal and documented) to the staff caring for the
patient or post procedure destination, as appropriate, and discuss with the patient
and / or person responsible where possible.
Specimens / images must be labelled correctly and labels checked with the patient
or person responsible or checked with another clinician where possible.
Arrange post procedure tests where clinically relevant.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 9 of 31
3 LEVEL 2 PROCEDURES
Definition
Examples
c
Requirements
Pre-procedure (including
Team Time Out)
Post procedure
- Proceduralist often
supported by an
assisting
proceduralist/s
- Usually requires
written consent
- Does not involve
procedural sedation
or general/regional
anaesthesia
- Usually performed
in wards,
emergency
departments,
clinics, imaging
departments,
interventional suites
- Lumbar puncture
- Insertion of chest
tube
- Ascitic tap
- Stress test
- Diagnostic
interventional
procedures
- Nuclear Medicine
therapies
- Non-superficial
biopsies
- IV or IT
administration of
chemotherapy
- IV administration
of contrast
- Centrally inserted
central venous
access device
8
STOP and confirm the
following before
commencing the
procedure
- Proceduralist/assisting
proceduralist/s introductions,
where appropriate
- Patient identification
- Procedure verification -
procedure + site/side/level,
where appropriate, matches
consent
- Patient position
- Essential imaging reviewed
- Allergy/adverse reaction
check
- Special medication/s
administered
- Antibiotics
- Implants and special
equipment
- Anticipated critical events
- Document procedure in the
patient’s health care record or
Radiology Information System
- Advice for clinical handover
- Equipment problems/issues
- Specimens/images labelled
correctly
- Post procedure tests where
clinically relevant e.g. CXR post
insertion of chest tube
3.1 Pre procedure (including Team Time Out)
Procedures not involving procedural sedation / anaesthesia are either Level 1 or Level
2 procedures. Refer to the definition and examples for guidance in classifying
procedures as Level 1 or Level 2.
The proceduralist, and where present assisting proceduralist/s, must STOP and
confirm the following minimum requirements immediately before commencing the
procedure. Where two or more staff members are involved they must introduce
themselves to each other, and the patient and their person responsible where
appropriate, by their preferred names and roles before the procedure commences.
3.1.1 Patient identification
The patient’s identity must be confirmed before any procedure commences.
Staff must confirm they have the correct patient by asking the patient, or their
person responsible, to state the patient’s full name and date of birth. Staff must not
c
The examples provided do not cover all possible procedures and the examples may be escalated to a higher level (ie Level 1
procedures may be classified by a health service as Level 2 or Level 3 procedures). Health services should consider development
of local lists of examples for Level 1, Level 2 and Level 3 procedures consistent with the requirements of this policy directive.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 10 of 31
state the patient’s name or date of birth and then ask the patient, or their person
responsible, if this information is correct.
The response must be confirmed against the details on the consent form / request
form / referral / treatment plan and patient identification band or approved patient
identification tool (including unique patient identifier), where appropriate.
Where patient details on the consent / request form / referral / treatment plan are
incomplete or there is a discrepancy with the information received from the patient,
or their person responsible, the correct information must be verified before
commencing the procedure and actions taken documented in the patient’s health
care record.
If the patient is unable to participate in the patient identification step, for example
due to physical incapacity, language issues, or is a child, and their person
responsible is not present, then the patient’s identification band or approved patient
identification tool (including unique patient identifier) should be used to confirm their
identification
3.1.2 Procedure verification
Consent must be obtained for any procedure as required by the NSW Health policy
directive on consent to medical treatment.
1
The consent form (where written consent obtained) must be completed as required
by the NSW Health policy on consent.
1
Request forms / referrals / treatment plans for procedures must include the
patient’s name, date of birth, sex and unique patient identifier (if available), and
should include the procedure site / side / level, reason for the procedure, details of
the examination / test/s required, the date the test/s were ordered, and the exact
anatomical location for the test/s.
Consent must be documented for high risk radiology and nuclear medicine
procedures for Diagnostic Imaging Accreditation Scheme (DIAS) accreditation.
9
The level of risk associated with each imaging procedure should be determined
locally based on the risk factors of the individual patient and the risk of the
procedure.
When contrast is used for procedures outside the operating theatre a patient
checklist that is specifically designed for contrast administration must be used.
The proceduralist must ask the patient, or their person responsible, to state what
procedure they understand will be performed and to state the site / side / level for
the procedure (where appropriate) and verify this matches the planned procedure
and consent / request form / referral / treatment plan.
9
Where procedure details on the consent form / request form / referral / treatment
plan are incomplete or there is a discrepancy the requesting clinician or a member
of their team must be contacted to clarify the information before commencing the
procedure and the response documented.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 11 of 31
3.1.3 Site / side / level marking
The site / side / level should be marked where there is the potential for error
involving multiple structures (fingers, toes, or lesions), left / right distinction, or
levels (spine).
The site /side / level marking for radiotherapy treatments involve the following.
- The mark should be on or near the incision site or radiotherapy site.
- For certain treatments the immobilising device may be marked.
- Site / side / level marking is not required in the following circumstances. For
multiple fractions of radiotherapy, where the site is usually only marked before
the first fraction and reapplied as necessary, and where markings are applied to
the immobilisation device rather than on the patient’s skin.
3.1.4 Patient position
The positioning of the patient must be verified as correct for the planned procedure.
The appropriate equipment for positioning and venous thromboembolism (VTE)
prophylaxis must be working and available for use during the procedure.
6
3.1.5 Essential imaging available
If imaging data are to be used to verify the procedure or site / side / level of the
procedure the proceduralist must verify in conjunction with the assisting
proceduralist/s, as appropriate, that:
The patient’s identity, the site of the procedure and the date of the image in relation
to the procedure all match.
The images are for the correct side of the body, oriented correctly, and correctly
labelled with the patient’s name and date of birth.
3.1.6 Allergy / adverse reaction check
The proceduralist should:
Ask the patient, or their person responsible, if they have a known allergy / adverse
reaction and if yes, what the allergy / adverse reaction was and what effect they
experienced. The response should be documented.
Check for any other source that may provide further information on allergies /
adverse reactions the patient might have e.g. treatment plan, progress notes.
Check that allergies / adverse reactions are noted on the allergy / adverse reaction
section of the National Inpatient Medication Chart or other relevant section of the
patient’s health care record.
Note that when contrast is used for procedures the allergy / adverse reaction check
must be included in a patient checklist that is specifically designed for contrast
administration.
Ensure the assisting proceduralist/s is aware of all identified allergies / adverse
reactions.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 12 of 31
3.1.7 Special medications administered
The proceduralist should confirm that any special medications required have been
administered.
3.1.8 Antibiotics
Antibiotic prophylaxis may be indicated and should be given in accordance with
current antibiotic therapeutic guidelines prior to the procedure commencing except
when antibiotics are withheld in order to get specimens for microbial testing.
3.1.9 Anticipated critical events
The proceduralist must consider, and discuss with the assisting clinician/s, the
planned procedure, critical steps, anticipated events and equipment requirements.
The proceduralist, and the assisting proceduralist/s, must verbally confirm sterility,
implants and equipment requirements.
3.2 Post procedure
3.2.1 Name of the proceduralist/s documented
The name of the proceduralist/s must be documented in the patient’s health care
record or Radiology Information System.
3.2.2 Name of the procedure documented
The proceduralist must confirm exactly what procedure was done, any expected or
unexpected adverse events and patient outcomes, and ensure this is documented
in the patient’s health care record or Radiology Information System. Where a
procedure has varied from that planned the rationale must be documented with
reason/s why.
3.2.3 Advice for clinical handover
Provide clinical handover advice (verbal and documented), including the patient’s
management plan post procedure, for the clinicians at the post procedure
destination and discuss with the patient and their person responsible where
possible.
Document and communicate any altered calling criteria on the relevant observation
chart.
3.2.4 Equipment problems / issues documented and advised to relevant staff
Malfunctioning equipment and instruments should be accurately identified to
prevent them from being used again until the problems are resolved. Any
equipment or instrument problems arising during the procedure must be
documented, and raised with the relevant staff so they can be resolved as soon as
possible. If an adverse event has occurred as a result of equipment / instrument
malfunction then this should be notified in the incident management system.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 13 of 31
3.2.5 Specimens / images labelled correctly
The proceduralist, and assisting proceduralist/s, must ensure the correct labelling
of any pathology specimen / images obtained during the procedure by verifying the
patient’s name, specimen / image description and any orienting marks.
3.2.6 Tests required
Referral for test/s post procedure should be discussed with the patient and their
person responsible where clinically appropriate, and arranged.
Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 14 of 31
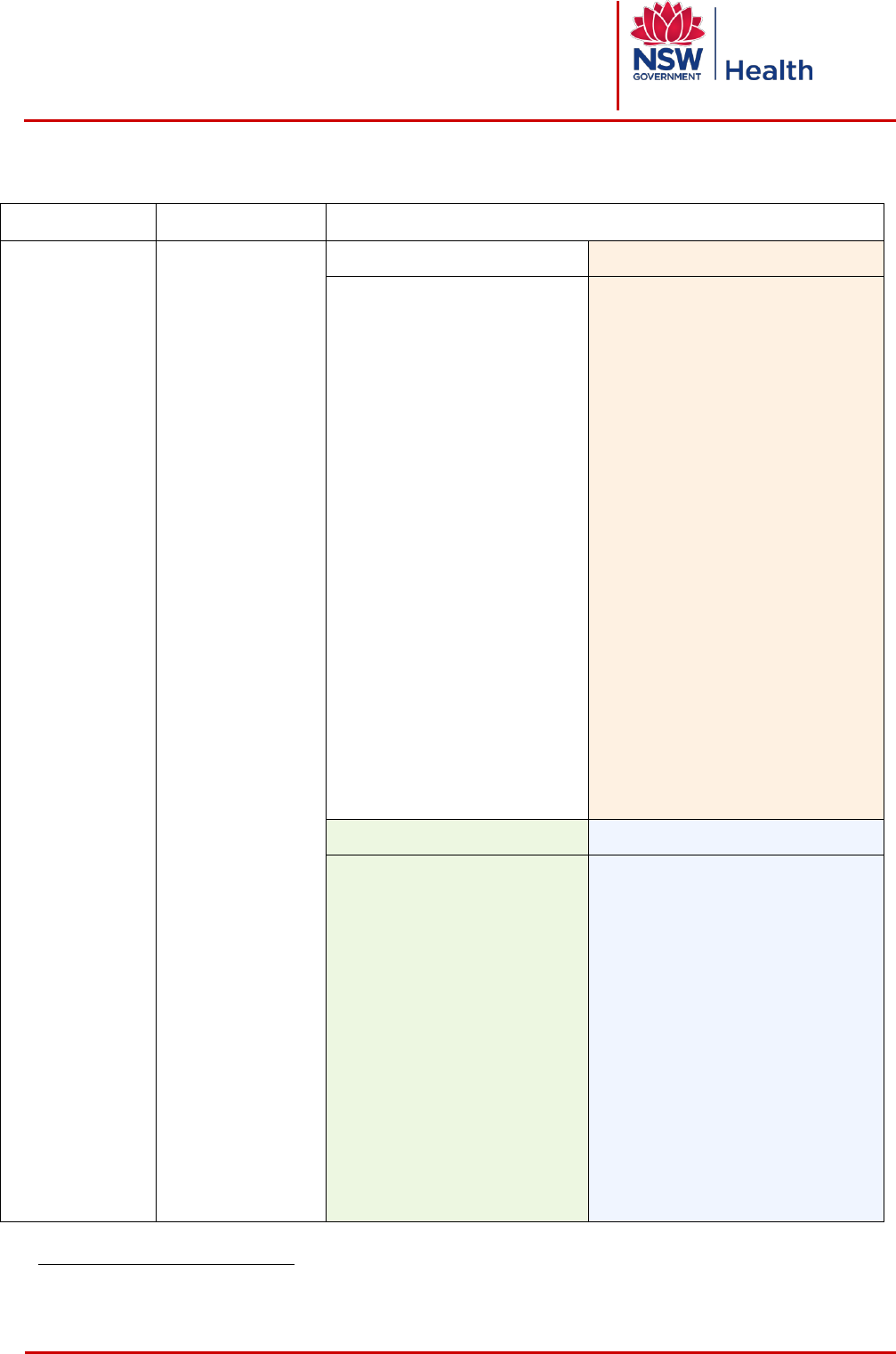
4 LEVEL 3 PROCEDURES
Definition
Examples
d
Requirements
- At least one
proceduralist
and a
procedural team
- Always requires
written consent
- Involves
procedural
sedation or
general /regional
anaesthesia
- Usually
performed in
formal
procedural
suites such as
operating
theatres,
emergency
departments,
endoscopy
suites,
interventional
imaging suites,
birthing suites,
cardiac
catheterisation
laboratories
- Surgical
procedure (OR)
- ECT
- Colonoscopy
- Bronchoscopy
- Interventional
imaging
procedure,
including:
•
Angiography
•
Cardiovascular
•
Coiling
•
Stenting
•
Interventional
Neuroradiology
1. Pre-procedure
2. Sign In
- Patient identification
- Procedure verification –
planned procedure +
site/side/level, where
appropriate, matches consent
- Site/side/level marking, where
appropriate
SIGN IN ONE
- Patient identification
- Procedure verification – planned
procedure + site/side/level, where
appropriate, matches consent
- Allergy/adverse reaction check
- Sedation/anaesthetic equipment
checked
- Patient sedation risk/anaesthetic
assessment
- Significant airway or aspiration risk
- Clinician airway monitor identified
- Clinician skilled to manage airway
identified
- Pulse oximeter working
- Risk of major bleeding
SIGN IN TWO
- Essential imaging available
- Site marking (exemptions)
- Implants and special equipment
- Proceduralist available to complete
procedure
3. Team Time Out
4. Sign Out
- Team member introductions
- Patient identification
- Procedure verification -
planned procedure +
site/side/level, where
appropriate, matches consent
- Patient position
- Essential imaging reviewed
- Allergy/adverse reaction
check
- Special medication/s
administered
- Antibiotics
- VTE prophylaxis
- Anticipated critical events
- Name of procedure recorded
- Counts/tray list checks correct
- Specimens/images labelled
correctly
- Blood loss documented; ongoing
blood loss discussed
- Equipment problems/issues
documented/ relevant staff
member advised or equipment /
instrument labelled
- Advice for clinical handover
d
The examples provided do not cover all possible procedures and the examples may be escalated to a higher level (ie Level 1
procedures may be classified by a health service as Level 2 or Level 3 procedures). Health services should consider development
of local lists of examples for Level 1, Level 2 and Level 3 procedures consistent with the requirements of this policy directive.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 15 of 31
Procedures involving procedural sedation / anaesthesia must always be classified as
Level 3 procedures.
4.1 Pre procedure requirements
The following must be undertaken before the patient is transferred to the procedural
suite.
4.1.1 Patient identification
The patient’s identity must be confirmed before any procedure commences.
Staff must confirm they have the correct patient by asking the patient, or their
person responsible, to state the patient’s full name and date of birth. Staff must not
state the patient’s name or date of birth and then ask the patient, or their person
responsible, if this information is correct.
The response must be confirmed against the details on the consent form / request
form / referral / treatment plan and patient identification band (including unique
patient identifier).
Where patient details on the consent form / request form / referral / treatment plan
are incomplete or there is a discrepancy with the information received from the
patient, or their person responsible, the correct information must be verified before
commencing the procedure and actions taken documented in the patient’s health
care record.
If the patient is unable to participate in the patient identification step, for example
due to physical incapacity, language issues, or is a child, and their person
responsible is not present, a member of staff from the preceding location of the
patient (e.g. ward or emergency department) must act as the patient’s advocate to
confirm the patient’s identity.
Patients undergoing Level 3 procedures must be wearing a patient identification
band.
10
4.1.2 Procedure verification
Consent must be obtained for all Level 3 procedures as required by the NSW
Health policy directive on consent to medical treatment.
1
The consent form must be completed as required by the NSW Health policy on
consent.
1
Request forms / referrals / treatment plans for procedures must include the
patient’s name, date of birth, sex and unique patient identifier and should include
the procedure site / side / level, reason for the procedure, details of the
examination / test/s required, the date the test/s were ordered, and the exact
anatomical location for the test/s.
Staff must ask the patient, or their person responsible, to state what procedure they
understand will be performed and to state the site / side / level for the procedure
(where appropriate) and verify this matches the planned procedure and consent
form / request form / referral / treatment plan.
9

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 16 of 31
Where procedure details on the consent form / request form / referral / treatment
plan are incomplete or there is a discrepancy the requesting clinician or a member
of their team must be contacted to amend or complete a new document before the
procedure commences and actions taken documented in the patient’s health care
record.
Verify x-ray and other imaging data are for the correct patient and are the correct
images, where appropriate.
Other relevant clinical information including documentation recorded electronically
must be available prior to the planned procedure.
Verification should be documented in the patient’s health care record, including a
record of individuals involved in the verification process.
4.1.3 Site / side / level marking
Site / side / level marking
Site / side / level marking is essential in cases where there is the potential for error
involving multiple structures (fingers, toes, or lesions), left / right distinction, or levels
(spine). In these cases, where appropriate, the site / side / level should be marked.
The site / side / level must be marked by one of the proceduralists (except for intra-ocular
surgery):
As a minimum, all cases involving multiple structures (fingers, toes or lesions),
laterality or levels (spine) must be marked.
Non-procedure sites / sides / levels must not be marked.
Marking occurs before the patient enters the procedural room, except in an
emergency.
The method of marking should be consistent throughout the organisation. Initials
must not be used in marking.
Marking takes place with the patient involved, awake and aware, where
appropriate. Note some paediatric, psychiatric and intellectually impaired patients
may find this distressing and marking may be done after these patients are
anaesthetised. For this group of patients it may be appropriate to have a person
responsible present.
The mark should be on or near the incision site.
The mark should be visible and sufficiently permanent so it remains visible
following skin preparation and draping.
The marking must be documented in the patient’s health care record by the person
marking the site / side / level.
Exception: For intra-ocular surgery where pre-operative mydriatic drops have been
ordered, the correct side may be marked by a registered nurse, and the marking
checked by a second registered nurse before the drops are given, in conjunction with
confirmation of the patient’s identity, checking of the consent, and verbal confirmation
by the patient, or their person responsible, of the side to have surgery. The mark

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 17 of 31
must be subsequently checked as the correct side for the procedure as required by
Sign In One, Sign In Two and Team Time Out.
Site / side / level marking exemptions
Site / side / level marking is not required in the following circumstances (although it can
be used):
To avoid confusion e.g. if a procedure requires a regional anaesthetic then only the
procedure site should be marked.
For single organ cases e.g. cardiac surgery, caesarean section.
Where the site of surgical entry is unambiguous e.g. midline incisions,
cystoscopies, laparoscopies.
If the site is obvious e.g. open trauma wound, large tumour.
For endoscopies.
For procedures where the catheter / instrument site is not predetermined e.g.
cardiac catheterisation, epidural / spinal analgesia / anaesthesia.
For radiology procedures where marking the site could add to the ambiguity of
subsequent procedures.
Where intra-procedure imaging for localisation e.g. radiological, MRI, stereotaxis,
ultrasound, radiation detection will be used.
Where the procedure site cannot be marked e.g. teeth, the site / side must be
clearly recorded in the patient’s health care record.
For premature infants, and some oral and maxillofacial surgery, where marking
may cause permanent marking of the tissues.
Where the patient refuses marking. Such refusal must be documented in the
patient’s health care record.
In a life-threatening emergency where the patient enters the procedural room
directly. This must be documented in the patient’s health care record.
4.2 Requirements for a Level 3 procedure checklist
There are three distinct stages to Level 3 procedure checklists with each stage
corresponding to a specific time period in the patient’s procedure.
Sign In
The period before commencing procedural sedation or
general / regional anaesthesia that is, immediately before
the procedural team prepares the patient for their procedure.
Sign In is further divided into two parts - Sign In One &
Sign In
Two
Team Time Out
The period immediately before commencing the procedure
to undertake a final patient identification and procedure
verification
Sign Out
The period before the patient / procedural team leave the
procedural area.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 18 of 31
A checklist must be used for every Level 3 procedure.
A checklist must include Sign In, Team Time Out and Sign Out.
Sign In One and Two may be combined with the agreement of sedationists /
anaesthetists and proceduralists.
The name of the clinician/s that completed each section of the checklist must be
clearly documented.
Section
Sign In One
Clinician responsible
Sedationist / Anaesthetist
Sign In Two
Proceduralist
Where Sign In One and Sign In Two are combined the names of both
clinicians responsible must be documented - that is the name of the
Sedationist / Anaesthetist and the name of the
Proceduralist
Team Time Out
Senior proceduralist
Sign Out
Nurse / Midwife
The checklist is part of the patient’s health care record.
The checklist must include confirmation of the patient’s identification and the
procedure verification.
The checklist should comply with the requirements of Sections 4.3 to 4.6 of this
policy.
For procedures performed outside an operating suite, Local Health Districts /
Specialty Health Networks (LHD / SHNs) may remove items included in a Level 3
procedure checklist, as set out in Sections 4.3 to 4.6, based on a risk management
approach considering issues such as the type of procedure and the procedural
setting. This would only apply when the items removed have no relevance to the
procedure being performed (e.g. for electroconvulsive therapy (ECT) procedures
the checklist might remove the items about blood loss or imaging). If modified
checklists are created then they must be clearly labelled with the location the
checklist will be used in or, if a procedure specific checklist, then the procedure
must be included in the title (e.g. ECT Procedure Safety Checklist).
Additional items not covered by this policy directive may be added as required.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 19 of 31
Checklists for Level 3 procedures must be approved by the LHD / SHN Chief
Executive or their delegate/s (such as Executive Directors for Clinical Governance,
Medical Services, Nursing & Midwifery) or the LHD / SHN’s quality and safety
committee. The approval must be documented.
4.3 Sign In One: Checklist completed by the sedationist / anaesthetist
Sign In One must be completed before commencing procedural sedation or general /
regional anaesthesia.
Sign In One is completed by the sedationist / anaesthetist in conjunction with another
member of the procedural team e.g. anaesthetic nurse / circulating nurse. Where there is
no sedationist / anaesthetist then a proceduralist must complete this check.
In procedural suites where a formal, documented verification check is performed prior to
entering the procedural suites e.g. in an airlock, theatre holding bay or reception area,
the Sign In One is an additional step that must occur in a room or area immediately
adjacent to the procedural room e.g. in the anaesthetic room if available, or in the
procedural room.
Sign In One must be completed before the patient enters the procedural room, except in
emergency situations, where an anaesthetic room does not exist or where the patient
enters the procedural room directly. In these cases Sign In One should be completed
inside the procedural room.
4.3.1 Patient identification
Patient identification must occur before any treatment / intervention is initiated
except if a life threatening or emergency situation exists.
Staff must ask the patient, or their person responsible, to state their full name and
date of birth. Staff must not state the patient’s name or date of birth and then ask
the patient, or their person responsible, if this information is correct.
The answers to these questions must be confirmed against the details on the
patient identification band. If there is a discrepancy between the details, the
procedure must not proceed until this is resolved.
If the patient is unable to participate in the final patient identification step prior to the
planned procedure/s, for example due to physical incapacity, language issues, or is
a child, then the patient’s person responsible or the patient’s identification band/s
should be used to confirm the patient’s identity.
4.3.2 Planned procedure matches consent
The consent form is the primary source of information about the patient’s planned
procedure. The procedure to be performed must match what has been written on
the patient’s signed consent form. Details on the consent form must be clear and
correct; and must match the health care record, the request / referral letter, the
patient’s or their person responsible’s, understanding of the procedure to be
undertaken and imaging data, where appropriate.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 20 of 31
A final consent check with the patient, or their person responsible, before sedating /
anaesthetising the patient gives the patient the opportunity to identify any mistakes.
If the planned procedure and consent do not match, the proceduralist must resolve
the matter before the patient receives procedural sedation / anaesthesia.
If the planned procedure information on the consent form is incorrect this should be
documented in the patient’s health care record as well as the actions taken to
resolve the discrepancy.
4.3.3 Site / side / level matches consent
The relevant team member should ask the patient, or their person responsible, to
state their site / side / level for the planned procedure. The team member must not
state the site / side / level for the planned procedure and then ask the patient, or
their person responsible, if this information is correct.
For some procedures (e.g. those that involve ovaries and fallopian tubes), side
detection may be unreliable preoperatively.
e
In these circumstances, side
verification is not recommended.
4.3.4 Allergy / adverse reaction check
The relevant team member should:
Ask the patient, or their person responsible, if they have a known allergy / adverse
reaction and if yes, what the allergy / adverse reaction was and what effect they
experienced.
Check for any other source that may provide further information on allergies /
adverse reactions the patient might have e.g. treatment plan, progress notes.
Check that allergies / adverse reactions are noted on the allergy / adverse reaction
section of the National Inpatient Medication Chart or other relevant section of the
patient’s health care record.
Note that when contrast is used for procedures the allergy / adverse reaction check
must be included in a patient checklist that is specifically designed for contrast
administration or a Level 3 checklist.
Ensure all team members are aware of all allergies / adverse reactions identified.
4.3.5 Sedation / anaesthetic equipment checked
When procedural sedation or anaesthesia is planned a formal check of the
necessary sedation / anaesthetic equipment must be completed prior to each
procedure to ensure the equipment is available and working. Continuous pulse
oximetry and blood pressure monitoring must be commenced on the patient prior to
commencing procedural sedation or anaesthesia and continued until the patient is
adequately recovered from this.
e
Gynaecology surgery for adnexal masses: it is not uncommon for a patient to be consented for a right sided procedure, based on
clinical examination or imaging (usually ultrasound) and to find at operation that the pathology is left sided (and vice versa). This is
due to the fact that the tubes and ovaries are lateral and posterior to the uterus and fall towards the midline of the pelvis, making it
easy to get the wrong side.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 21 of 31
4.3.6 Patient sedation risk / anaesthetic assessment done
When procedural sedation or anaesthesia is planned a medical assessment must
be completed prior to commencement of the procedure (except in a life threatening
emergency). This must include documentation of the patient’s medical condition/s
and their sedation risk / anaesthetic assessment. When a non-anaesthetist plans to
give procedural sedation an assessment must be made as to whether an
anaesthetist is required to assess and manage the patient. This decision must be
documented in the patient’s health care record.
4.3.7 Significant airway risk
When procedural sedation or anaesthesia is planned the sedationist / anaesthetist
must formally assess the patient’s airway and document this in the patient’s health
care record prior to commencing procedural sedation / anaesthesia. If this
assessment indicates a significant airway risk then an anaesthetist must be present
before sedation is given.
When a significant airway risk is identified the procedural sedation / anaesthesia
must not commence until all required special equipment needed is present and
functional, and procedural team members needed are present.
Functioning and clean suction equipment must always be immediately available
when procedural sedation / anaesthesia is given.
4.3.8 Significant aspiration risk
The risk of aspiration should also be evaluated and documented. If the patient has
symptomatic active reflux or a full stomach, the sedationist / anaesthetist must
consider what additional steps might be taken to reduce the increased risk of
aspiration.
When a significant aspiration risk is identified the procedural sedation / anaesthesia
must not commence until all required special equipment needed is present and
functional, and the appropriate procedural team members are present.
Functioning and clean suction equipment must always be immediately available.
4.3.9 Identification of clinician airway monitor and availability of skilled personnel
When procedural sedation is to be used, and where an anaesthetist is not present
to care exclusively for the patient, a clinician airway monitor other than the
proceduralist must be nominated whose primary responsibility is to monitor the
patient’s level of consciousness and to monitor and provide the initial management
of cardio-respiratory status of the patient during the procedure. There must be
present a clinician skilled in airway management and cardio-pulmonary
resuscitation relevant to the patient’s age.
4.3.10 Risk of major bleeding
Defined as the risk of bleeding more than:
500 ml of blood for adults
7 ml / kg of blood for children

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 22 of 31
>750 ml of blood for maternity patients.
11
If there is a risk of major bleeding:
The procedural team should confirm there is a valid group and screening available.
If antibodies are present and the blood bank indicates that this may delay the
provision of cross-matched blood, then at least two units of compatible cross-
matched blood should be available before proceeding.
The patient should have large bore venous access.
Intra-procedure blood loss should be measured and the patient monitored for signs
of hypovolaemia.
4.4 Sign In Two: Checklist completed by the proceduralist
Sign In Two must be completed before commencing procedural sedation or general /
regional anaesthesia.
Sign In Two must be completed by a proceduralist who is required to confirm the
following.
4.4.1 Essential imaging available
If imaging data are to be used to verify the site or procedure, a proceduralist must
confirm with another member of the procedural team that:
Images are correct and properly labelled for the correct side of the body, oriented
correctly, and labelled with the patient’s name and date of birth.
Patient’s identity, the site of the procedure and the date of the image, in relation to
the procedure, all match.
4.4.2 Site marked
A proceduralist must confirm that the site has been marked or marking is not required
(Refer to Section 4.1.3 Site marking).
4.4.3 Implants and special equipment
If any implant (type / side / size / power) and / or special equipment is required, its
availability and function where possible to check, must be checked by two team
members.
A proceduralist must be present prior to commencement of procedural sedation /
anaesthesia to confirm that sterile instrumentation, implants and / or any special
equipment required are present and functional.
Where an implant is used the product’s label, code reference and serial number
should be recorded in the patient’s health care record.
4.4.4 A proceduralist who can complete the procedure is immediately available
Confirm that a proceduralist, who can complete the procedure is immediately
available before the patient receives procedural sedation / anaesthesia and before
moving to the Team Time Out stage.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 23 of 31
4.5 Team Time Out – Checklist led by the senior proceduralist
Team Time Out is the final patient safety check and must occur immediately before the
procedure commences in the room where the procedure is to be conducted. Usually this
will be after procedural sedation / anaesthesia has commenced. The senior proceduralist
present must lead the Team Time Out. The proceduralist, sedationist / anaesthetist and
other members of the procedural team must ALL confer and agree on all aspects of the
Team Time Out section of the checklist.
Success of Team Time Out is reliant on active communication amongst all members of
the procedural team. It is the responsibility of the senior proceduralist present to ensure
that Team Time Out is completed. The procedure should not commence until all team
members are satisfied that the patient identification and procedure verification processes
have been completed and patient identification and procedure verification are correct.
Each and every member of the procedural team is responsible for ensuring Team
Time Out occurs and for raising any concerns they may have during Team Time
Out.
Where discrepancies are noted or disagreements occur at Team Time Out, the
procedure must be delayed until the issues are resolved. Only for reasons of clinical
urgency should the procedure commence. The justification for proceeding in the
presence of such discrepancies must be documented by the proceduralist in the patient’s
health care record as soon as the procedure is completed and an incident report must
also be completed.
Where previous identification / verification steps have occurred satisfactorily but a
discrepancy in information or disagreement in identification / verification occurs at Team
Time Out, an incident report should also be completed even if the issues are resolved
satisfactorily.
If disagreement occurs in an extreme emergency situation, the senior member of the
procedural team is responsible for the care of the patient and should decide the most
appropriate course of action.
Only after Team Time Out has been completed should the procedure commence.
4.5.1 Procedural team member introductions
All procedural team members must introduce themselves to each other by their
preferred names and roles before the procedure commences. Team members may
change frequently and it is important in effective management that all team
members understand who each member is and their role.
In situations where multiple patient procedures are undertaken consecutively and
there is no change in team members during the list, then this action can occur at
the commencement of the list.
In addition, teams may adopt local strategies such as documenting the name and
role of team members on a whiteboard.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 24 of 31
4.5.2 Patient identity
The patient’s identity must be confirmed against approved patient identifiers,
including the patient identification band/s, consent and documentation. The
identification band/s used for confirmation must be accessible after positioning and
draping.
4.5.3 Planned procedure matches consent
The consent form is the primary source of information about the patient’s planned
procedure. The planned procedure must be matched against the patient’s consent
form and imaging data, where appropriate.
The processes described in this policy directive should not preclude the use of
discretion by the treating proceduralist to alter the procedure for reasons of clinical
judgement. However, significant changes to the documented procedure must be
communicated to all members of the procedural team and must be recorded in the
patient’s health care record.
4.5.4 Site / side / level mark matches consent
The site / side / level mark must be consistent with the site / side / level
documented in the consent and imaging.
For some procedures (e.g. those involving ovaries and fallopian tubes), side
detection may be unreliable preoperatively.
f
In these circumstances, side
verification is not recommended (Refer to Section 4.3.3 Site / side / level matches
consent).
4.5.5 Patient position
The positioning of the patient must be confirmed as correct for the planned
procedure and site / side / level.
4.5.6 Essential imaging reviewed
One of the proceduralists must confirm that the essential imaging is in the
procedural area and ready for use during the procedure. If imaging data are used
to verify the site or procedure, the proceduralist must review and confirm the
images are correct and properly labelled. If essential images are not available,
the proceduralist must decide if it is safe to proceed and document this decision
in the patient’s health care record.
4.5.7 Allergies / adverse reactions
Confirm any known allergies / adverse reactions. This will raise the team’s
awareness of precautions that may need to be taken during the procedure to
avoid allergies / adverse reactions.
f
Gynaecology surgery for adnexal masses: it is not uncommon for a patient to be consented for a right sided procedure, based on
clinical examination or imaging (usually ultrasound) and to find at operation that the pathology is left sided (and vice versa). This is
due to the fact that the tubes and ovaries are lateral and posterior to the uterus and fall towards the midline of the pelvis, making it
easy to get the wrong side.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 25 of 31
4.5.8 Special medications administered
Confirm that any special medications required (e.g. eye drops, steroids, mannitol)
have been administered.
4.5.9 Antibiotics
Antibiotic prophylaxis is considered best practice for a number of complex
procedures. Where ordered, antibiotic prophylaxis must be given prior to the
procedure (ideally within 60 minutes of the procedure commencing).
12
Antibiotics for caesarean sections may be given prior to the procedure or after the
cord is clamped. This should be determined by local procedures or by the senior
proceduralist. The senior proceduralist must decide the timing of antibiotic
administration for a caesarean section and document this decision in the patient’s
health care record.
An exception is when antibiotics are withheld in order to obtain specimens for
microbial testing or to observe the patient.
4.5.10 VTE prophylaxis
The need for VTE prophylaxis must be assessed on every patient. Where
indicated, it should be commenced prior to the procedure. Methods include
anticoagulants, compression stockings and foot / calf compressors. Indicators for
use are outlined in the NSW Health policy directive on prevention of venous
thromboembolism.
6
Note that not all VTE prophylaxis methods will commence
pre-procedure e.g. anticoagulants may commence post procedure.
4.5.11 Anticipated critical events
Effective team communication reduces error, prevents major complications and
supports efficient teamwork. To ensure the procedural team has a common
understanding of the planned procedure and expected outcomes / issues:
The proceduralist must verbally brief the team on the planned procedure, critical
steps, anticipated events and equipment requirements.
The sedationist / anaesthetist must verbally identify any specific patient or
procedure concerns they have.
The nurse / midwife verbally confirms that
- Any required equipment is available and, where possible to check, functional
- Any required items or implants are available and, if necessary, sterilised /
disinfected.
4.6 Sign Out – Checklist completed by the nurse / midwife
Sign Out should occur before the patient / procedural team leave the procedural area.
Sign Out is designed to ensure that all relevant patient documentation is completed and
that appropriate clinical handover can be conducted. The nurse / midwife is responsible
for Sign Out and should complete this section before the patient / procedural team leave

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 26 of 31
the procedural area. The proceduralist or sedationist / anaesthetist could also complete
this section.
Responsibility for documentation must be consistent with the requirements set out in the
NSW Health policy directive on handling instruments and accountable items which says
that “while documentation is primarily completed by the circulating nurse / midwife, the
instrument nurse / midwife is ultimately responsible for ensuring the completion and
accuracy of all documentation relating to the surgery/procedure. The anaesthetic nurse is
responsible for documenting the anaesthetic nursing care provided.”
13
The nurse / midwife confirms the following.
4.6.1 Name of the procedure recorded
The proceduralist must document the procedure that was carried out in the
patient’s health care record. Where a procedure has varied from what was
planned the rationale must be also noted in the health care record.
4.6.2 Count / tray list checks
To ensure there are no instruments, accountable items or other items
unintentionally retained in the patient, a count / tray list check must be performed
as required by the NSW Health policy directive on handling instruments and
accountable items.
14
This is usually attended prior to the patient leaving the procedure room. However,
for the management of multiple or complex instrument trays, for example, the
policy directive says that “the final instrument checks may be completed
immediately post procedure and before the next patient enters the operating or
procedure room”.
15
4.6.3 Specimens / images labelled correctly
The proceduralist and another member of the procedural team must ensure the
correct labelling of any pathology specimen / images obtained during the
procedure by verifying the patient’s name, specimen / image description and any
orienting marks.
4.6.4 Equipment problems / issues documented and advised to relevant staff
Malfunctioning equipment and instruments need to be accurately identified, and if
possible isolated from other equipment and instruments, to prevent them from
being used again until the problem/s is resolved. Any equipment or instrument
problem/s arising during the procedure must be documented, raised with the
relevant staff or the equipment / instrument labelled so the problem/s can be
resolved as soon as possible. If an adverse event has occurred as a result of
equipment / instrument malfunction then this should be notified in the incident
management system.
The procedural team confirms the following.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 27 of 31
4.6.5 Blood loss documented, ongoing blood loss discussed
To ensure that early warning signs of blood loss can be assessed, the blood loss
(if any) during the procedure should be documented and any anticipated post
procedure bleeding discussed. If significant post procedure bleeding is
anticipated, blood loss criteria for notifying medical staff must be documented.
4.6.6 Advice for clinical handover
The following advice for clinical handover (verbal and documented) must be provided to
staff at the post procedure destination.
The procedural team has discussed the patient management plan for recovery,
post procedure investigations and communication. This is expected to include any
key messages that should be relayed to the patient or their person responsible.
Any altered calling criteria documented if patient is not being recovered in a Post
Anaesthetic Care Unit (PACU) or Recovery.
Post procedure VTE prophylaxis has been ordered, if required.
Post procedure care should be discussed with the patient, or their person
responsible, where possible.
5 INCIDENTS
In the event of an incident:
If the patient’s condition permits, an immediate plan to rectify the error/s should
be made by the senior member of the procedural team. Wherever possible, the
patient and their person responsible should be involved in the management plan
Manage incidents as required by NSW Health policy directives on incident
management and open disclosure.
16
Serious incidents must be discussed at appropriate patient safety or clinical
review meetings. Local improvement strategies should be developed in response
to these serious incidents
Report to the Special Committee Investigating Deaths Under Anaesthesia
(SCIDUA) even when anaesthesia / sedation did not contribute, regardless of
cause of death.
17
6 AUDITING AND REPORTING
Auditing of compliance with this policy directive must be undertaken by each LHD/SHN.
Performance indicators may be included in quarterly reporting to LHD / SHN clinical
councils.

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 28 of 31
7 RESOURCES
Resources to support implementation of this policy directive can be found at the
following sites.
Clinical Procedure Safety
http://www.cec.health.nsw.gov.au/programs/clinical-procedure-safety
This site includes a checklist for Medical Imaging Departments (Radiology and Nuclear
Medicine) which has been developed by clinicians of the Agency for Clinical
Innovation’s Radiology and Nuclear Medicine Networks.
Safe Sedation
https://www.aci.health.nsw.gov.au/resources/anaesthesia-perioperative-
care/sedation/safe-sedation-resources
8 ABBREVIATIONS
ECT
Electroconvulsive therapy
LA
Local anaesthetic
IDC
Indwelling catheter
MRI
Magnetic resonance imaging
IV
Intravenous
NGT
Nasogastric tube
IT
Intrathecal
VTE
Venous thromboembolism

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 29 of 31
9 REFERENCES
1. Consent to Medical Treatment - Patient Information, PD2005_406 at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2005_406.pdf
2. Clinical Handover is defined at https://www.aci.health.nsw.gov.au/resources/acute-
care
3. Incident is defined in Incident Management Policy, PD2014_004 at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_004.pdf
4. Client Registration Policy, PD2007_094
(http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2007_094.pdf)
Client Registration Guideline, GL2007_024
(http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2007_024.pdf)
Patient Identification Bands, PD2014_024
(http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_024.pdf).
5. ANZCA, PS09 – Guidelines on Sedation and/or Analgesia for Diagnostic and
Interventional Medical, Dental or Surgical Procedures, 2014 at
http://www.anzca.edu.au/resources/professional-documents .
6. Prevention of Venous Thromboembolism, PD2014_032 at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_032.pdf
7. Refer to Consent to Medical Treatment - Patient Information, PD2005_406 for
information about how long a consent remains valid and who should obtain consent at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2005_406.pdf
8. Central Venous Access Device Insertion and Post Insertion Care, PD2011_060 at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2011_060.pdf
9. Diagnostic Imaging Accreditation Scheme: Practice Accreditation Standards,
Australian Government Department of Health
http://www.health.gov.au/internet/main/publishing.nsf/Content/diagnosticimaging-accred2
10. Patient Identification Bands, PD2014_024 at
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_024.pdf
11. WHO guidelines for safe surgery:2009 : safe surgery saves lives at
http://www.who.int/patientsafety/safesurgery/tools_resources/9789241598552/en/
12. Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory
statement from the National Surgical Infection Prevention Project. Clinical Infectious
Diseases, 2004;38:1706–15.
13. Refer to section 4.2 of Management of Instruments, Accountable Items and Other
Items used for Surgery or Procedures, PD2013_054
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2013_054.pdf

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 30 of 31
14. Management of Instruments, Accountable Items and Other Items used for Surgery or
Procedures, PD2013_054
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2013_054.pdf
15. Refer to section 5.5 of Management of Instruments, Accountable Items and Other
Items used for Surgery or Procedures, PD2013_054
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2013_054.pdf
16. Incident Management, PD2014_004
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_004.pdf
Open Disclosure Policy, PD2014_028
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_028.pdf
17. Special Committee Investigating Deaths Under Anaesthesia (SCIDUA)
http://www.cec.health.nsw.gov.au/incident-management/mortality-review-
committees/scidua

Clinical Procedure Safety
PROCEDURES
PD2017_032
Issue date: September-2017
Page 31 of 31
10 FURTHER READING
Agency for Clinical Innovation, Anaesthesia Perioperative Care Resources
http://www.aci.health.nsw.gov.au/resources/anaesthesia-perioperative-care
Antibiotic Therapeutic Guidelines - available via the CIAP website (“Medications” then
“Therapeutic Guidelines eTG”) at http://www.ciap.health.nsw.gov.au/home.html or
directly at accessible at
https://tgldcdp.tg.org.au.acs.hcn.com.au/topicTeaser?guidelinePage=Antibiotic&etgAc
cess=true
ANZCA, PS09 – Guidelines on Sedation and/or Analgesia for Diagnostic and
Interventional Medical, Dental or Surgical Procedures, 2014
http://www.anzca.edu.au/resources/professional-documents.
Collaborating Hospitals' Audit of Surgical Mortality (CHASM)
http://www.cec.health.nsw.gov.au/incident-management/mortality-review-
committees/chasm
Maternity – Breast Milk: Safe Management, PD2010_019
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2010_019.pdf
National Safety and Quality Health Service Standards, Australian Commission on
Safety and Quality in Health Care https://www.safetyandquality.gov.au/our-
work/assessment-to-the-nsqhs-standards/
Infants and Children: Management of Acute and Procedural Pain in the Emergency
Department, GL2016_009
http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2016_009.pdf
Royal Australasian College of Surgeons (RACS), Surgical Safety Checklist, October 2009
www.surgeons.org/media/12661/LST_2009_Surgical_Safety_Check_List_(Australia_and
_New_Zealand).pdf
Royal Australian and New Zealand College of Radiologists. Iodinated Contrast Media
Guideline, 2016 Version
http://www.ranzcr.edu.au/quality-a-safety/radiology/iodinated-contrast-media-guideline
World Health Organization, Surgical Safety Checklist, 2008
http://www.who.int/patientsafety/safesurgery/ss_checklist/en/
