
Sample Final Examination
Organic Chemistry I
CHEM 2423
O
H
HO
H
HO
OH
O
H
H
H
H
OH
Practice Exam A

2
Name ___________________________________________
CHEMISTRY 2423 Practice FINAL EXAM A
DIRECTIONS: A periodic table is attached at the end of this exam. Please answer all questions as
completely and clearly as possible, showing all your work.
Part I. Nomenclature and Structures (2 points each)
1. Give the correct IUPAC name for the following structures (2 pts each):
(a)
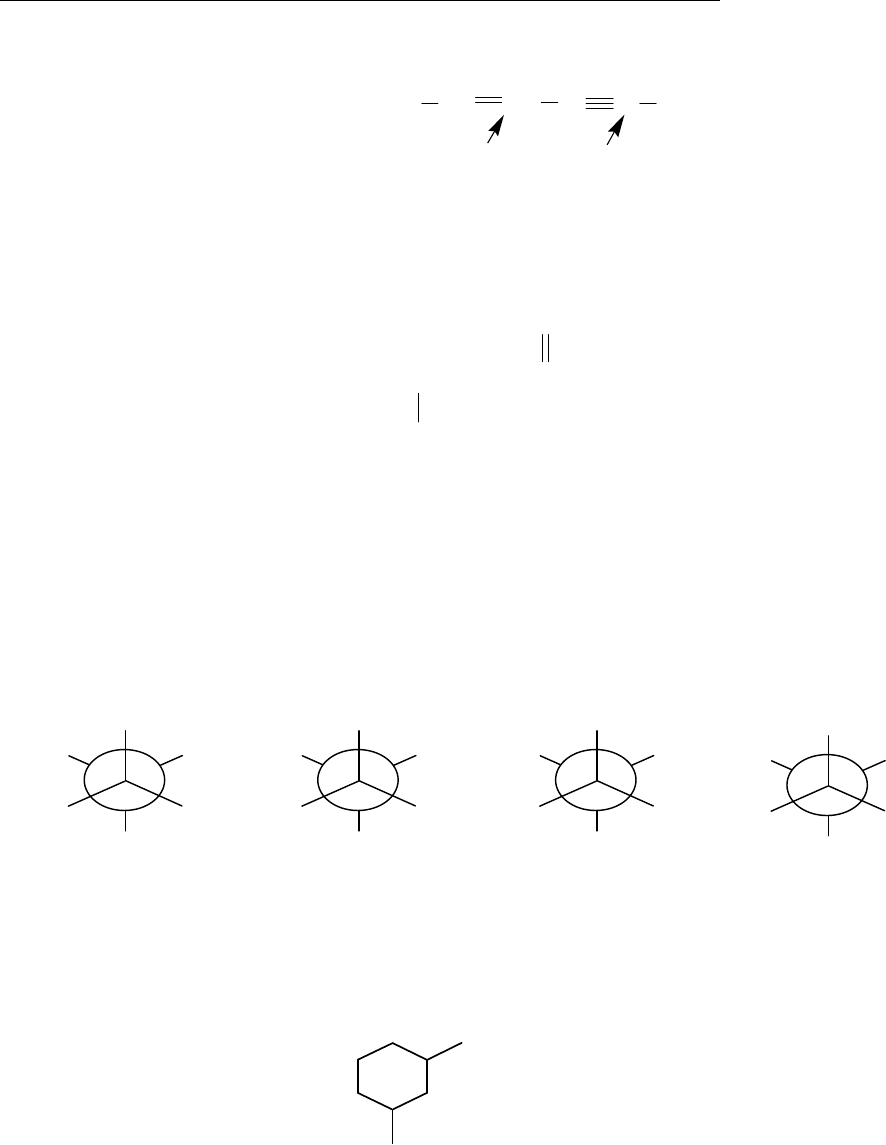
(b)
(c)
Br
Br
Br
C = C
H
H
CH
3
CH(CH
3
)
2
(a) _______________________________________________________________
(b) _______________________________________________________________
(c) _______________________________________________________________
2. Draw the structure that corresponds to the following name (2 pts each):
(a) (S)-2-bromobutane (b) (E)-3-iodo-2-pentene (c) 2,2-dimethyl-3-hexyne
3
Part II. Multiple choice. Circle the one best answer. (2 points each)
3. What is the correct hybridization for the indicated carbon atoms.
CH
3
CH CH C C CH
3
1 2
(A) sp
2
, sp (B) sp
3
, sp (C) sp
3
, sp
2
(D) sp, sp
2
4. What functional groups are present in the following molecule?
CH
3
- CH - CH
2
- C- OH
O
OH
(A) alcohol and carboxylic acid (B) ketone and ether
(C) alcohol and ester (D) ketone and aldehyde
5. Which one of the following structures is the most stable conformation for 2-bromo butane
2
-C
3
bond axis)
B r
H
C H
3
H
H
C H
3
B
r
B r
H
C H
3
C H
3
C H
3
C H
3
C H
3
C H
3
C H
3
H
H
H
H
H
H
B r
H
H
(A) (B) (C) (D)
6. Identify the number of primary, secondary, and tertiary carbons, respectively, in the
following molecule:
(A) 1, 3, 1 (B) 4, 1, 1 (C) 2, 4, 2 (D) 2, 2, 4
7. What is the conjugate acid for CH
3
OH?
(A) CH
3
O
-
(B) CH
2
OH
-
(C) CH
3
-
(D) CH
3
OH
2
+

4
8. Describe the indicated C-H bond indicated below in terms of orbital overlap:
CH
3
-CH=CH
2
(A) sp
3
-sp
3
(B) sp
3
-sp
2
(C) sp
2
-1s (D) sp-1s
9. Which one of the following groups has the highest priority?
C
O
O
CH
3
C
O
H
C
O
OH
C
CH
2
OH
(A) (B) (C) (D)
10. Rank the following according to the most acidic to least acidic.
I) HCl II) CF
3
-COOH III) CH
3
-COOH
(A) I>II>III (B) II>I>III (C) III>II>I (D) II>III>I
11. What is the relationship between the two compounds:
a n d
(A) enantiomers (B) diastereomers (C) same molecule (D) none of these
12. Which reaction intermediate is formed by the following reaction?
Br
2
/ CCl
4
Br
Br
Br
Br
Br
(A) (B) (C) (D)
13. Which one of the following alkenes is the most stable?
(A) (B) (C) (D)
CH
2
CCH
3
CH
2
CH
3
CH
3
C C
CH
3
CH
3
CH
3
CH
3
C C
CH
3
H
CH
3
H
C C
CH
2
H
CH
3
CH
3

5
14. Which one of the following carbocation intermediates is the least stable?
A B
C
D
15. Which one of the following substances is referred to as a Gilman reagent?
(A) CH
3
I (B) (CH
3
)
2
CuLi (C) CH
3
MgBr (D) CH
3
Li
16. An allylic hydrogen is indicated at which position in the structure below?
CH
3
- HC = CH – C C - H
(A) 1
(B) 2
(C) 3 1 2 3 4
(D) 4
17. Which one of the following diagrams correctly illustrates the displacement of bromine
N
2 reaction?
(A) (B) (C) (D)
Br CH
3
HO
-
CH
3
Br
HO
-
CH
3
Br
HO
-
HO
-
Br CH
3
18. Inversion of configuration results from which one of the following mechanisms?
(A) E1 (B) E2 (C) S
N
1 (D) S
N
2
19. Which one of the following molecules has the highest boiling point?
(A) CH
3
CH
2
CH
3
(B) CH
3
CH
2
COCH
3
(C) CH
3
CH
2
CH
2
OCH
3
(D) CH
3
CH
2
CH
2
OH
20. What sequence correctly descrides the steps involved in a radical chain reaction?
I) initiation II) termination III) propagation
(A) I, III, II (B) I,II,III (C) III, I, II (D) none of these

6
21. Which of the following are examples of
syn
addition to an alkene?
(A) hydrogenation and hydration (B) hydrobromination and hydroboration
(C) hydration and hydrobromination(D) hydrogenation and hydroboration
22. Which one of the following hydrogens is the most acidic?
CH
3
- HC = CH – C C - H
(A) 1
(B) 2
(C) 3 1 2 3 4
(D) 4
23. The following process demonstrates:
OH
O
(A) resonance (B) conjugation (C) racemization (D) tautomerism
24. What reagent is needed to convert 1-hexyne to 2-hexanone?
(A) O
3
/Zn/ H
3
O
+
(B) PCC (pyridinium chlorochromate)
(C) BH
3
/H
2
O
2
/OH
-
(D) HgSO
4
/ H
2
SO
4
/ H
2
O
25. In mass spectroscopy,
(A) the sample is irradiated with infrared radiation
(B) the heat of combustion of the sample is measured
(C) the sample is bombarded with a stream of high energy electrons
(D) the sample is irradiated with ultraviolet radiation
26. Select the structure of a compound with the molecular formula C
6
H
14
which has a
base peak at m/e = 57 in the mass spectrum.
(A) CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
(B) (CH
3
)
2
CHCH
2
CH
2
CH
3
(C) (CH
3
)
3
CCH
2
CH
3
(D) (CH
3
)
2
CHCH(CH
3
)
2

7
Part III. Reactions (2 points each)
Give the major product(s) of each of the following reactions. Show all relevant stereochemistry. (2
pts each)
Br
2
/hv
CH
3
C
H
3
27.
CHCH
2
CH
3
28.
+ Br
2
CCl
4
29. CH
3
C CH + HCl
e
t
h
e
r
(1 eq)
30.
Br
KOH
+
31.
NBS
32.
1) O
3
2) Zn/H
3
O
33.
CH
2
I
2
/ Zn(Cu)
34.
1) BH
3
, THF
2) H
2
O
2
, H
35.
CH
3
- CH
2
- C C Na
1) CH
3
Br
2) H
2
/ Lindlar catalyst
36.
C
H
=
C
H
-
C
C
-
H
excess H
2
Pd / C

8
Part IV. Synthesis (3 points each)
Show by a series of reactions how you could prepare the following compounds from the indicated
starting compound. Be sure to clearly indicate the reagent used in each step.
37.
O
38.
C = C
C = C
CH
3
CH
3
CH
3
CH
3
HH
H
H
39.
C
H
2
C
H
3

9
Part V. Mechanisms (3 points each)
Write a complete mechanism for the following reactions. Show all intermediate structures, formal
charges, and electron flow using the curved arrow convention.
(3 pts each)
40.
C
H
3
B
r
1) KOH
2) HBr
CH
3
Br
41.
OH
CH
3
H
2
SO
4
Heat
C
H
3

10
Part VI. Spectra (5 points)
Use the mass spectrum for a hydrocarbon shown below to answer questions 42 - 44.
42. What is the base peak (1 pt)?
43. What is the parent ion peak (1 pt)?
44. What is the structure of the compound (3 pts)?

11
CHEMISTRY 2423 Practice FINAL EXAM A (Answers)
PART I. (2 points each)
1. (a) 4-bromo-3-ethylheptane
(b) 1,3-dibromo-1,3-cyclohexadiene
(c) trans-4-methyl-2-pentene
2.
Br
H
CH
2
CH
3
(a)
C = C
CH
3
H
I
CH
2
CH
3
(b)
CH
3
- C - C C - CH
2
- CH
3
CH
3
C
H
3
CH
3
(c)
PART II. (2 points each)
3. A 4. A 5. B 6. C 7. D 8. C 9. A
10. A 11. C 12. D 13. B 14. B 15. B 16. A
17. B 18. D 19. D 20. A 21. D 22. D 23. D
24. D 25. C 26. C
PART III. (2 points each)
CH
3
- C - CH
2
- CH
3
CH
3
B
r
or others ( R &S)
27.
28.
Br
Br
29.
C = C
H
H
CH
3
Cl
30.
CH
3
+
major
CH
2
minor
31.
CH
3
Br
CH
3
+
C
H
3
CH
3
Br

12
32. H - C - CH
2
- CH
2
- CH
2
- C - H
O
O
33.
3
4
.
OH
35.
C = C
H
H
CH
3
CH
2
CH
3
36.
C
H
2
C
H
2
C
H
2
C
H
3
PART IV. (3 points each)
37.
O
B r
2
C H - C H
2
- B r
B r
C
C - H
H g S O
4
, H
2
O
H
2
S O
4
2 K O H
C C l
4
38.
C = C
C = C
C H
3
C H
3
C H
3
C H
3
HH
H
H
B r
2
C H
3
- C H - C H - C H
3
B
r
B r
2 K O H
C H
3
- C
C - C H
3
H
2
/ L idlar cataly st
39.
C
H
2
C
H
3
H I
I
( C H
3
C H
2
)
2
C u L i

13
PART V. (3 points each)
40.
H
CH
3
Br
H
OH
CH
3
H
+ H
2
O +
AND
CH
3
H
H - Br
CH
3
H
H
CH
3
Br
::
..
..
..
-
:Br
..
..
::
-
Br
:
41.
O- H
CH
3
CH
3
H
+ -
OHSO
3
O - H
H
CH
3
H
:OHSO
3
+H
2
SO
4
E
1
CH
3
PART IV. (5 points) (1+1+3)
42. m/e
-
= 43 43. m/e
-
= 72
44.
C
H
3
-
C
H
-
C
H
2
-
C
H
3
CH
3
