
BRC GLOBAL STANDARD
AGENTS AND BROKERS ISSUE 2
FSMA GUIDANCE
AND
PREPAREDNESS
MODULE FOR
AGENTS AND
BROKERS
(applicable to importers of record, retailers and manufacturing sourcing)

1
CONTENTS
The Brokers role and FSMA ............................................................................................................................ 2
Suppliers and GFSI benchmarked certifications ............................................................................................ 2
How to use this module .................................................................................................................................... 2
6 Requirements of the FSMA Preparedness for Agents and Brokers Module ............................................... 4
6.1 Preventive Controls for Human Food .................................................................................................... 4
6.2 Preventive Controls for Animal Food ..................................................................................................... 5
6.3 Food Defense ........................................................................................................................................... 5
6.4 Supplier Verification Program ............................................................................................................... 6
6.5 Sanitary Transportation ....................................................................................................................... 13
Appendix 1 ..................................................................................................................................................... 17
Glossary .......................................................................................................................................................... 18
FSMA References ........................................................................................................................................... 20
Legislation .................................................................................................................................................. 20
Guidance ..................................................................................................................................................... 20
Training ...................................................................................................................................................... 20

2
FSMA Preparedness for Agents and Brokers Module
The Brokers role and FSMA
At time of writing, the regulatory requirements for the role of agent or broker are not clear (with the
exception of the importer of record). By definition, the agent or broker does not “produce or hold” product,
therefor many of the regulations do not apply directly to their actions.
That being said, the role of the agent or broker is an important one, and just as a broker acts as a
facilitator to the movement of product between producer source and customer, BRC has recognized the
equally important role the broker has in supplier approval for those customers. Hence this module is
designed to a certain degree to show where the regulations may impact the activity of the broker or agent,
but primarily designed to allow the agent or broker to be a source for appropriate supplier approval
information to move from the producer to customer. In the age of FSMA, it is no longer acceptable to
facilitate product movement, the agent or broker must facilitate product movement and proof of
compliance. Most of the requirements are laid out in such a way so that the broker uses it as part of their
supplier approval, and is able to provide that supplier approval evidence to their customers. This way a
broker can be closer to a “complete solution” to their customers.
Suppliers and GFSI benchmarked certifications
It is widely recognized that a GFSI benchmarked certification is a solid mechanism to assess compliance
to most requirements within FSMA regulations. It is also recognized that at the time of writing, no
scheme in and of itself fully meets the requirements prescriptively. Where a GFSI benchmarked scheme
has an additional module to cover gaps or prescriptively identify evidence of compliance, this should be
used. Where none exists, additional evidence may be required, depending on the gaps between the scheme
requirements and FSMA. To obtain information on the BRC Food Global Standard FSMA Preparedness
Addendum for you suppliers, contact enquiries@BRCGlobalStandards.com
How to use this module
This module supports agents and brokers in understanding how new regulations established under the
U.S. Food Safety Modernization Act (FSMA) apply to the company’s business activities. It is divided into
four parts, which includes the Checklist, Appendix 1, Glossary, and References. The checklist identifies
those legislative requirements across the suite of FSMA rules, which BRC recommends that—all agents

3
and brokers selling or trading food in the U.S. –establish and implement as a part of their food safety and
quality management system for stability and transparency in supply chain operations.
The checklist addresses compliance requirements across the following FSMA rules, which have
applicability to various types of agent and broker operations.
Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for
Human Food
Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for
Food for Animals
Foreign Supplier Verification Programs for Importers of Food for Humans and Animals
Mitigation Strategies to Protect Food Against Intentional Adulteration
Sanitary Transportation of Human and Animal Food
The Foreign Supplier Verification Program and Sanitary Transportation rules have the greatest
applicability to most agent and broker operations, thus the BRC has identified the prescriptive elements
of these rules in the checklist to support agents and brokers in regulatory compliance.
It is important to
note that the BRC has established requirements of the FSVP rule, which are in addition to those required
by the BRC Global Standard for Agents and Brokers, as best practice for all agents and brokers and does
limit these requirements to U.S. importers as defined by the regulation
. In creating this module, the BRC
aims to support agents and brokers in risk management and regulatory compliance when purchasing or
trading food products sold in the U.S. regardless of whether they are sourced from a foreign or domestic
(U.S. based) supplier.
Satisfying the requirements of either checklist does not guarantee compliance with U.S. legislation.
Rather, compliance with the module provides clear guidance to help agents and brokers navigate the suite
of FSMA rules. Examples provided throughout this module are for illustrative purposes to aid in the
interpretation of BRC requirements and do not authoritatively determine the regulation or exclusion of
agent and broker activities. Full regulatory compliance with FSMA legislation is the responsibility of the
company.
Appendix 1 correlates some agent and broker activities with FSMA legislation to help agents and brokers
determine which FSMA rules regulate their business activities. Key terms used in the following checklist
are defined in the Glossary to aid in interpretation of module requirements. Additionally, a list of
legislative and training references are provided to support agents and brokers in regulatory compliance.

4
6 Requirements of the FSMA Preparedness for Agents and Brokers Module
Clause
Checklist Item
Guidance
6.1 Preventive Controls for Human Food
Does this section apply to me?
Preventive Controls regulation is applicable to product suppliers, importers of record, and potentially to others in
scope.
Any facility that produces or holds product destined for the U.S. is generally required to register with the FDA as
a food facility, and re-register every 2 years.
For clarification, Google “registration of food facilities – FDA” or refer to guidance documents listed in the
References section of the Standard.
If yes, consider the following:
Statement of Intent: The company shall assess its business operations for the need to comply with U.S. regulation
21 CFR part 117 and document compliance where required.
6.1
The company shall comply
with U.S. regulation 21
CFR part 117:
Current
Good Manufacturing
Practice, Hazard Analysis,
and Risk-Based Preventive
Controls for Human Food
where the company
additionally manufactures,
processes, packs, or holds
food for sale in the United
States and is required to
register as a food facility
per section 415 of the U.S.
Food, Drug & Cosmetic Act
(FD&C).
The company shall determine if it is required to register with the U.S.
Food and Drug Administration (FDA) as a food facility according to
legislative requirements set forth in FD&C, section 415. This applies to
domestic (U.S. based) and foreign companies. Where a company is based
outside of the U.S. territory and is required to register with FDA as a food
facility due to business operations, the company must designate a U.S.
representative.
This process of determining the need to meet regulations is equally
required for the suppliers to the broker or agent.
Where a company determines it must register as a food facility with the
FDA, it must then determine if its business operations are regulated by
21 CFR part 117 (Preventive Controls for Human Food rule). For
example, where an agent or broker holds (or manages the holding of) food
products covered under the scope of the rule in a company owned
warehouse, the company is required to comply and establish a written
hazard analysis, written preventive controls and written monitoring,
corrective action and verification procedures.
The scope of Preventive Controls for Human Food requirements are
covered in the BRC Global Standard for Food Safety and accompanying
FSMA Preventive Controls Preparedness Module. Additionally,
certification to the BRC Global Standard for Storage and Distribution
may support in achieving partial compliance with 21 CFR part 117 for
storage and distribution facilities.
Regulatory Reference: 21 CFR part 117

5
6.2 Preventive Controls for Animal Food
Does this section apply to me?
Preventive Controls regulation is applicable to product suppliers, importers of record, and potentially to others in
scope.
Any facility that produces or holds product destined for the U.S. is generally required to register with the FDA as
a food facility, and re-register every 2 years.
For clarification, Google “registration of food facilities – FDA” or refer to guidance documents listed in the
References section of the Standard.
If yes, consider the following:
Statement of Intent: The company shall assess its business operations for the need to comply with U.S. regulation
21 CFR part 507 and document compliance where required.
6.2
The company shall comply
with U.S. regulation 21
CFR part 507:
Current
Good Manufacturing
Practice, Hazard Analysis,
and Risk-Based Preventive
Controls for Food for
Animals
where the
company additionally
manufactures, processes,
packs, or holds food for
sale in the United States
and is required to register
as a food facility per
section 415 of the U.S.
Food, Drug & Cosmetic Act
(FD&C).
The company shall determine if it is required to register with the U.S.
Food and Drug Administration (FDA) as a food facility according to
legislative requirements set forth in FD&C, section 415. This applies to
domestic (U.S. based) and foreign companies. Where a company is based
outside of the U.S. territory and is required to register with FDA as a food
facility due to business operations, the company must designate a U.S.
representative.
Where a company determines it must register as a food facility with the
FDA, it must then determine if its business operations are regulated by
21 CFR part 507 (Preventive Controls for Animal Food rule). For
example, where an agent or broker holds pet food products covered under
the scope of the rule in a company owned warehouse, the company is
required to comply and establish a written hazard analysis, written
preventive controls and written monitoring, corrective action and
verification procedures.
The scope of Preventive Controls for Animal Food requirements are
primarily covered in the BRC Global Standard for Food Safety and
accompanying FSMA Preventive Controls Preparedness Module.
Additionally, certification to the BRC Global Standard for Storage and
Distribution may support in achieving partial compliance with 21 CFR
part 507 for storage and distribution facilities.
Regulatory Reference: 21 CFR part 507
6.3 Food Defense
Does this section apply to me?
This clause is applicable to product suppliers, importers of record, and potentially to others in scope.
If yes, consider the following:
Statement of Intent: The company shall assess its business operations for the need to comply with U.S. regulation
21 CFR part 121 and document compliance where required.
6.3
The company shall comply
with U.S. regulation 21
CFR part 121:
Mitigation
Strategies to Protect Food
The company shall determine if it is required to register with the U.S.
Food and Drug Administration (FDA) as a food facility according to
legislative requirements set forth in FD&C, section 415. This applies to
domestic (U.S. based) and foreign companies. Where a company is based

6
Against Intentional
Adulteration
where the
company additionally
manufactures, processes,
packs, or holds food for
sale in the United States
and is required to register
as a food facility per
section 415 of the U.S.
Food, Drug & Cosmetic Act
(FD&C).
outside of the U.S. territory and is required to register with FDA as a food
facility due to business operations, the company must designate a U.S.
representative.
Where a company determines it must register as a food facility with the
FDA, it must then determine if its business operations are regulated by
21 CFR part 121 (Food Defense rule). For example, the company shall
develop and implement a written food defense plan for holding food stored
in liquid storage tanks but is excluded from this requirement for other
holding activities. Additionally, the requirement to develop a food defense
plan does not apply to packing and labeling activities where the container
in direct contact with the food remains intact nor does it apply to food for
animals.
Certification to the BRC Global Standard for Food Safety or Storage and
Distribution may support in achieving partial compliance with 21 CFR
part 121.
Regulatory Reference: 21 CFR part 121
6.4 Supplier Verification Program
Does this section apply to me?
This clause is applicable to all agents and brokers, importers of record, and potentially to others in scope.
If yes, consider the following:
Statement of Intent: The company shall establish a Supplier Verification Program to cover the scope of all
suppliers (foreign and domestic) used to source food for sale in the U.S., which is compliant with the requirements
of 21 CFR part 1 subpart L.
Of particular note to brokers dealing with dietary supplements and their components:
The FSVP rule has some modified requirements for agents and brokers purchasing or trading dietary
supplements. This is defined in section 1.511 of the rule. It basically requires same provisions outlined in section
6.4 below but requires compliance with cGMP’s as specified by 21 CFR part 111.
6.4.1
The company shall ensure
that all suppliers used to
source food sold in the U.S.
produce such food in
compliance with sections
402, 403(w), 418, and 419
of the U.S. Food, Drug &
Cosmetic Act (FD&C).
FD&C, section 402 defines adulterated food.
FD&C, section 403(w) defines misbranded food.
FD&C, section 418 requires that owners, operators or agents in charge of
registered food facilities evaluate food hazards, apply preventive controls
to ensure food is not adulterated or misbranded, and monitor the
performance of applied controls.
FD&C, section 419 requires farms that produce and harvest raw
agricultural commodities (fruits and vegetables) to develop and
implement procedures, practices and processes to minimize the risk of
food hazards and ensure produce is not adulterated.
Supplier compliance with U.S. legislation shall be documented through a
Supplier Verification Program, written assurances and other applicable
methods.
Regulatory Reference: 21 CFR part 1 subpart L (1.502)
6.4.2
One or more Qualified
Individuals shall be
identified on the
Qualified Individuals are responsible for performing all activities of the
Supplier Verification Program. This includes but is not limited to:
7
organization chart with
responsibility for activities
of the company’s Supplier
Verification Program.
Qualified Individuals with
responsibility for record
review must read and
understand the language
of the record requiring
review.
For importers of record, a
Qualified Individual is
mandatory. It is
recommended that all
companies dealing in the
US appoint and support a
Qualified Individual
within the organization.
Suppliers that process or
hold product, must have a
Preventative Controls
Qualified Individual.
Establishing the Supplier Verification Program in compliance
with the requirements of this Standard
Conducting a written hazard analysis in accordance with clause
6.4.5
Conducting and reviewing supplier risk assessments in
accordance with clauses 6.4.6 and 6.4.7
Ensuring the use of approved or temporary suppliers in
accordance with clause 6.4.8
Establishing and conducting supplier verification activities in
accordance with clause 6.4.9 – 6.4.11
Maintaining written assurance from subsequent manufacturers
regarding the control of hazards in compliance with clause 6.4.12
Ensuring hazard labeling in accordance with clause 6.4.12
Conducting a review of the Supplier Verification Program and
updating in accordance with clause 6.4.13
Documenting changes to the Supplier Verification Program in
accordance with clause 6.4.14
Recordkeeping activities in accordance with clause 6.4.15
Regulatory Reference: 21 CFR part 1 subpart L (1.503a)
6.4.3
Each designated Qualified
Individual shall have
completed the Food Safety
Preventive Controls
Alliance (FSPCA) Foreign
Supplier Verification
Program training course. A
record documenting
completion of the course
must be maintained.
Qualified Individuals shall have completed the approved FDA training
course for developing and implementing a Foreign Supplier Verification
Program.
While this training course focuses on specific regulatory requirements for
importers, the BRC recommends this course for all Qualified Individuals
complying with the requirements of this module as it describes how to
conduct a hazard analysis, supplier risk assessment and determine
appropriate supplier verification activities for the sourcing of food
products compliant with U.S. legislation.
Where the agent or broker processes or holds product, they will
additionally need to designate a PCQI who has taken the FSPCA training
for Preventive Controls and may need to take Food Defense training (e.g.,
if they process food or hold bulk liquid). Note: food defense training and a
food defense plan would not be required if the company only holds
packaged product.
Regulatory Reference: 21 CFR part 1 subpart L (1.503a)
6.4.4
A Qualified Auditor,
independent of the
supplier, shall be
responsible for conducting
an onsite audit where this
is performed as a
verification activity of the
company’s Supplier
Verification Program.
A Qualified Auditor shall be a trained and experienced food safety auditor
with knowledge of the food category being evaluated, the requirements of
this Standard and applicable U.S. food legislation.
Where an onsite audit is an established verification activity for a
supplier, the Qualified Auditor shall conduct the assessment on behalf of
the company. Examples of qualified auditors may be registered auditors
with GFSI benchmarked schemes, government inspectors or
appropriately trained and experienced second-party auditors.

8
While not mandatory—but strongly recommended based on current
interpretations of FSMA regulation—company managed auditors should
receive the Qualified Individual training noted in clause 6.4.3 in addition
to qualifications in audit technique and category risk assessment.
Regulatory Reference: 21 CFR part 1 subpart L (1.503b)
6.4.5
Conduct a written hazard
analysis for each food type
purchased or traded by the
company, which is sold in
the U.S., to identify known
or reasonably foreseeable
hazards associated with
the food or facility. This
includes:
Naturally occurring
hazards
Economic adulterants
which affect food safety
Environmental
pathogens where
ready-to-eat (RTE) food
is exposed to the
environment prior to
packaging and the
packaged food does not
receive a kill step
Radiological hazards
Unintentional
adulterants which
affect food safety
A Qualified Individual is responsible for conducting a written hazard
analysis, which identifies and evaluates all known or reasonably
foreseeable hazards for each food type purchased or traded by the
company where that food is offered for sale in the U.S.
Similar to Codex Alimentarius HACCP methodology, the hazard analysis
must include an assessment of the severity of illness or injury and
likelihood of occurrence if the hazard were to occur in the absence of
CCP’s and/or preventive controls.
The hazard analysis must consider known or reasonably foreseeable
hazards in all
Materials (or material groups)
Process steps
Production environment
Supply and distribution chain activities
Intended and reasonably foreseeable use
Other related activities
A company’s existing hazard analysis—developed in compliance with
section 2 of this Standard—should be reevaluated in consideration of the
provisions of this module requirement for regulatory compliance. For
example, a company may need to integrate or cross-reference material
risk assessments, supplier risk assessments and/or security risk
assessments into the hazard analysis.
The company may utilize a supplier’s hazard analysis provided that it
was conducted by a Qualified Individual as defined in clauses 6.4.2 and
6.4.3 of this Standard. The company is responsible for reviewing and
assessing the supplier’s hazard analysis for integration into its own.
Examples of naturally occurring hazards include heavy metals or
mycotoxins. Unintentionally introduced hazards may refer to pathogen
cross-contamination or allergen cross-contact. Intentionally introduced
hazards for economic gain include economically motivated adulterants
(EMA’s) such as melamine or other harmful substitution ingredients (e.g.,
industrial oil, wood pulp, etc.). Radiological hazards must be identified
and evaluated where there is a known prevalence in the raw material or
ingredient due to sourcing from a susceptible region or where materials or
the food product has the potential to be contaminated (e.g., from water
sources in susceptible areas)
Additionally, the hazard analysis must evaluate environmental
pathogens where a ready-to-eat (RTE) food is exposed to the environment
prior to packaging and the packaged food does not receive a kill step to
eliminate or significantly minimize the pathogen. Examples of
environmental pathogens include
Salmonella
spp. (typically found in dry
processing environments) and
Listeria monocytogenes
(common in wet

9
processing environments) although these pathogens are generally
ubiquitous in food handling and processing environments.
Regulatory Reference: 21 CFR part 1 subpart L (1.504)
6.4.6
Conduct and document a
supplier risk assessment,
which evaluates a
supplier’s performance and
risk posed by the food.
Supplier approval criteria
as required by clause 4.1.1
and supplier verification
activities as required by
clause 6.4.10 of this
Standard shall be based on
the supplier risk
assessment required by
this clause.
A Qualified Individual is responsible for conducting a written supplier
risk assessment in compliance with clause 4.1.1 of this Standard, which
shall additionally include an evaluation of
Hazards requiring a control
Entity administering the control
Supplier’s food safety procedures and practices
Supplier performance based on food safety history
FDA regulatory compliance
Storage and transportation practices
The company may utilize an established risk assessment for a given
supplier to conduct the supplier risk assessment required by this clause
provided that it was conducted by an entity other than the foreign
supplier (i.e., no self-evaluation) and performed by a Qualified Individual
as defined in clauses 6.4.2 and 6.4.3 of this Standard. The company is
responsible for reviewing and assessing the established risk assessment
for integration into its own supplier risk assessment.
The supplier risk assessment shall serve as the basis for determining
supplier approval and appropriate supplier verification activities
(including frequency) as required by the applicable requirements of this
Standard.
Regulatory Reference: 21 CFR part 1 subpart L (1.505a)
6.4.7
Review the supplier risk
assessment when new
information about risk
assessment criteria raises
additional concerns as to
the safety or legality of the
food or, at a minimum,
every 3 years if new
information does not
trigger a review. Document
each review of the supplier
risk assessment.
A Qualified Individual is responsible for reevaluating the supplier risk
assessment when new information raises additional concerns about one or
more suppliers and the food produced.
For example, the supplier risk assessment shall be reevaluated where a
new hazard requiring a control emerges for a food purchased or traded by
the company. Alternatively, it shall be reevaluated where the supplier
has received an FDA warning letter, import alert or other food safety
compliance action from another country’s food safety agency.
Additionally, it may need to be reevaluated where an onsite verification
audit reveals changes to a food safety procedure or practice resulting in
audit non-conformities.
Where new information changes a supplier’s risk ranking, this shall
prompt the company to reconsider the supplier’s approval status and
whether verification activities and frequency need to be changed.
At a minimum, the supplier risk assessment shall be reevaluated every 3
years where new information does not trigger an earlier review.
Regulatory Reference: 21 CFR part 1 subpart L (1.505c)
6.4.8
The company shall
purchase or trade food
from an approved supplier
according to the supplier
approval procedure
established for compliance
The supplier approval procedure shall describe the process and
responsibility for approving suppliers and ensuring that the company
only purchases from approved suppliers.
The procedure shall additionally describe the process and responsibility
for using unapproved suppliers by exception on a temporary basis, which

10
with clauses 4.1.1 and
4.1.6 of this Standard.
Where a temporary
situation requires the use
of an unapproved supplier,
the approval procedure
shall document the process
and conditions for this
exception, which shall
include adequate
verification before trading,
purchasing or importing.
Use of the supplier
approval procedure shall
be documented.
shall include the requirement for conducting supplier verification
activities before purchasing or trading.
Use of the supplier approval procedure shall be recorded by a Qualified
Individual.
Regulatory Reference: 21 CFR part 1 subpart L (1.506a)
6.4.9
Establish and implement a
written procedure(s),
which describes the
process for ensuring
supplier verification
activities are conducted to
provide assurance that
hazards are significantly
minimized or prevented.
The supplier verification procedure shall describe the process and
responsibility for ensuring that the following activities are conducted for
all suppliers:
Use of approved suppliers based on the outcome of a supplier risk
assessment
Determination of supplier verification activities, which provide
assurance that hazards are significantly minimized or prevented
Determination of verification activities and frequency based on
the outcome of a supplier risk assessment
Implementation of verification activities before purchasing or
trading
Implementation of verification activities at the determined
frequency following initial use of supplier
Recordkeeping requirements for all supplier verification activities
Documented processes for supplier verification activities required by
clauses 4.1.2, 4.1.5, 4.4.1, and 4.5.1 of this Standard should be
reevaluated in consideration of the provisions of this module requirement
for regulatory compliance. For example, a company may need to integrate
or cross-reference its procedures for ongoing review of supplier
performance, verification of buying specs through product sampling and
testing, and other verification activities which are conducted to ensure
the legality of products in the country of sale.
Regulatory Reference: 21 CFR part 1 subpart L (1.506b-c)
6.4.10
Determine and document
appropriate verification
activities for each supplier
before trading, purchasing
or importing and
periodically thereafter.
Verification activities must
include one or more of the
following commensurate
with the level of risk
determined in the supplier
risk assessment:
A Qualified Individual is responsible for determining and documenting
verification activities for each supplier based on risk.
Initial and ongoing supplier approval activities established for compliance
with clause 4.1.2 of this Standard may be identified as supplier
verification activities where all conditions of clause 6.4.11 are met. An
example of this would be certification of the supplier to a GFSI
benchmarked scheme, which includes assessment of regulatory
compliance with applicable FDA regulation (e.g., Preventive Controls or
Produce Safety rules).
Additionally, ongoing review criteria established in clause 4.1.5, product
sampling and testing established in clause 4.4.1, and/or activities which
verify the legality of products established in clause 4.5.1 may be used as

11
Onsite audit by a
Qualified Auditor
Product sampling and
testing
Review of supplier's
food safety records
Other appropriate
activities, which verify
that hazards are
controlled and are
based on the supplier’s
performance and risks
associated with the
food
For serious hazards, an
annual onsite audit is
required. Serious hazard
typically includes ready-to-
eat products, as well as
any product or commodity
that has been linked to a
health incident in the U.S.
in the past.
verification activities where all conditions of clause 6.4.11 are met. An
example of this would be lot or batch specific testing for a hazard
controlled by the supplier (e.g.,
Salmonella
) according to a statistically
valid sampling plan for detection of the hazard where present.
Other verification activities more appropriate to ensure hazards are
effectively controlled by the supplier or less frequent audits may be
applied where documented justification is provided.
Regulatory Reference: 21 CFR part 1 subpart L (1.506d)
6.4.11
Conduct verification
activities initially and
periodically thereafter
according to the following
criteria.
(1) Onsite audits shall be
performed by a Qualified
Auditor and include
assessment of the food
safety plan and compliance
with FDA regulation
where applicable.
(2) Product sampling and
testing shall identify the
lot or batch, number of
samples, method of
analysis, results, and
laboratory conducting the
test.
(3) Record review shall
pertain to relevant records
of the supplier’s food safety
system with documented
conclusions.
(4) Verification activities
may not be performed by
the supplier with the
Supplier verification shall be conducted by a Qualified Individual
according to the activity and frequency determined in 6.4.10.
Verification activity results—whether conducted by the company or
another entity—shall be promptly reviewed and assessed by a Qualified
Individual. Where results do not provide assurance as to the safety of the
food, corrective action shall be applied in accordance with clauses 3.9.1
and 6.4.13 of this Standard. Qualified Individuals conducting or
reviewing verification activities shall be free of financial conflict of
interest.
For onsite audits, a record of the audit report to include date, auditing
firm, Qualified Auditor, results, non-conformity and associated corrective
action shall be maintained by the company. FDA inspection results—or
regulatory inspection by a country whose food safety system is recognized
as equivalent by FDA—may suffice for an annual audit.
Product sampling and testing shall meet the requirements of section 4.4
of this Standard in addition to ensuring documentation (e.g., Certificates
of Analysis) conforms to the requirements of this clause. Where a hazard
is detected through product testing, corrective action shall be linked to
the out-of-specification result.
Each review of a supplier’s food safety records shall be documented and
identify the date, records reviewed, outcome, and applicable corrective
actions.
Where other verification activities are utilized, records of the activity
shall be maintained.
Regulatory Reference: 21 CFR part 1 subpart L (1.506e)

12
exception of sampling and
testing.
(5) Qualified Individuals
shall conduct or review
verification activities.
6.4.12
Where a known or
reasonably foreseeable
hazard is not controlled by
the supplier, the company
shall have annual written
assurance from a
subsequent manufacturer
that they (or a downstream
party) are responsible for
controlling a hazard in
compliance with
Preventive Controls or
other applicable
legislation.
Where the hazard is
subsequently controlled,
product documents used
for sale, trade or
importation, shall identify
the food as "not processed
to control [identified
hazard]’’.
This clause requires the hazard analysis to look “forward” into the supply
chain, document and communication hazards past to customers.
One example would be buying and selling unpasteurized tree nuts. The
hazard of pathogens is identified, but if not controlled (via some form of
pathogen reduction such as pasteurization) the customer’s responsibility
for managing the risk must be identified.
Where the company purchases or trades product from a supplier that does
not control an identified hazard because it is controlled downstream in
the supply chain by a manufacturer, the company shall acquire written
assurance from its customer that they (or a subsequent entity) take
responsibility for applying appropriate controls prevent or minimize the
hazard in compliance with applicable FDA regulation.
Written assurance provided by the company shall include the following
elements and be reissued annually.
Effective date
Description of the assurance including a description of procedures
for preventing or minimizing the hazard
Printed names and signatures of authorized individuals
In addition to documenting written assurance for the control of hazards,
the company shall ensure that product documents (e.g., purchase orders,
contracts, bill of lading, shipping manifest, etc.) clearly identify the
product as "not processed to control [identified hazard]’’.
Regulatory Reference: 21 CFR part 1 subpart L (1.507)
6.4.13
The corrective action
procedure shall include
review of the Supplier
Verification Program and
modification where
necessary when a system
non-conformity identifies
that a supplier does not
produce food in compliance
with the requirements of
this module.
The corrective action procedure established in compliance with clause
3.9.1 of this Standard shall additionally include the requirement for the
company to review its Supplier Verification Program where a non-
conformity is the result of a failure by the supplier to produce food in
compliance with sections 402, 403(w), 418, and 419 of the U.S. Food, Drug
& Cosmetic Act (FD&C).
Review of the Supplier Verification Program as a part of the corrective
action process shall be performed by a Qualified Individual and
documented. Modification to the Supplier Verification Program shall be
considered to prevent recurrence of the non-conformity and may include
additional or more frequent verification activities or alternatively,
supplier disapproval.
The corrective action process shall consider the company’s obligations
under U.S. law relating to product recalls, which shall be performed in
accordance with section 3.11 of this Standard.
Regulatory Reference: 21 CFR part 1 subpart L (1.508)

13
6.4.14
The Supplier Verification
Program shall be signed
and dated upon initial
completion and following
any changes.
A Qualified Individual is responsible for signing and dating the Supplier
Verification Program upon completing and following any changes.
Regulatory Reference: 21 CFR part 1 subpart L (1.510)
6.4.15
The company shall keep all
records related to the
Supplier Verification
Program as original or
electronic records for a
minimum of 2 years.
retrievable within 24
hours. English translations
shall be provided upon
request.
Records relating to the
company's processes and
procedures (e.g., approval
procedure or verification
activities) shall be retained
for 2 years after
discontinued use.
This recordkeeping requirement expands clause 3.3.2 of this Standard for
all documents, procedures and records related to the Supplier Verification
Program. This shall include but is not limited to:
Hazard analysis
Supplier approval procedure
Supplier risk assessment
Supplier verification procedure
Supplier contracts
Records of onsite audits (including the review of audit reports)
Records of sampling and testing (including the review of COA’s or
laboratory reports)
Records pertaining to the review of a supplier’s food safety records
Records of other supplier verification activities
Records pertaining to the review and modification of the Supplier
Verification Program
Corrective action procedure and records
Written assurance of hazard control
Training records for Qualified Individuals
Electronic records are defined as onsite.
Regulatory Reference: 21 CFR part 1 subpart L (1.510)
6.5 Sanitary Transportation
Does this section apply to me?
This section applies to suppliers and agents or brokers who arrange transportation or receive product.
If yes, consider the following:
Statement of Intent: The company shall ensure sanitary transportation practices for food transported by motor or
rail vehicle within the U.S., which are compliant with the requirements of 21 CFR part 1 subpart O.
6.5.1
The company shall ensure
that contracts with U.S.
shippers, receivers,
loaders, and carriers
specify their responsibility
for compliance with
FSMA’s Sanitary
Transportation rule.
Where the company acts as
the shipper or receiver, it
shall ensure compliance
with the rule.
Responsibilities shall
ensure transportation
operations are conducted
in a manner to prevent
Agents and brokers who arrange for the transportation of food in the U.S.
are defined as "shippers" in the Sanitary Transportation rule.
Agents and brokers who receive food at any point in the U.S. after ground
transportation—even where they are not the final recipient—are defined
as "receiver" in the Sanitary Transportation rule.
Examples of conditions and controls to prevent food from becoming unsafe
include: segregation, isolation, packaging, hand washing, and
temperature control. The type of food must be considered when
establishing transport conditions and controls.
Where controls fail and food may be rendered unsafe, the shipper,
receiver, loader, or carrier shall not sell or distribute food. They are
responsible for communicating the failure to other parties to ensure food
is not sold unless a Qualified Individual determines that the control
deviation did not render the food unsafe.

14
food from becoming unsafe
during transport (i.e.,
apply controls) and that
responsibility for
compliance with the
regulation is assigned to
competent supervisory
personnel.
Regulatory Reference: 21 CFR part 1 subpart O (1.908a)
6.5.2
Where the company
arranges transportation, it
shall document sanitary
design requirements and
cleaning procedures of
vehicles appropriate for
the type of food to be
transported. These
requirements shall be
communicated to the
loader and carrier.
Where the company does
not arrange
transportation, the above
provision shall be
documented in the
shipping service contract
to ensure the shipper
documents sanitary
specifications of vehicles
for the loader and carrier,
which are appropriate for
the type of food.
The shipper shall specify in writing and communicate to the U.S. carrier
or loader, sanitary specifications for ensuring vehicles and equipment are
in an appropriate sanitary condition for the transport of food. This shall
include sanitary design requirements and cleaning practices, which are
appropriate for the type of food.
Examples of sanitary specifications may include:
Specifying the temperature and any requirements for a pre-
cooling phase where temperature controlled product is shipped
Procedures for ensuring that the previous load does not cross-
contaminate food where bulk food is transported
Procedures for cleaning, sanitizing and inspecting vehicles
Segregation methods to prevent cross-contamination or cross-
contact
Regulatory Reference: 21 CFR part 1 subpart O (1.908b)
6.5.3
Contracts with loaders
shall specify that the
loader is responsible for
following sanitary
specifications provided by
shipper.
Contracts with U.S. loaders shall provide agreement between the
company and its service provider that loaders shall follow written
sanitary specifications from the shipper regardless of whether the shipper
is the company or another entity. This shall supplement the requirements
of clause 4.2.3 of this Standard.
Regulatory Reference: 21 CFR part 1 subpart O (1.908c)
6.5.4
Where the company
receives temperature
controlled product
immediately following
transportation, it shall
conduct an assessment to
determine whether the
food was subject to
temperature abuse.
Where the company receives temperature controlled product, they are
responsible for assessing and recording whether the food was subject to
temperature abuse.
The assessment may include:
Measuring product temperature upon unloading
Documenting the refrigeration unit’s temperature setting
Measuring ambient temperature of the container or refrigeration
unit holding product
Evaluating product for physical and quality indicators of
temperature abuse (e.g., warm product, off-odors, etc.)

15
Where the company’s customer or another entity receives the product,
they bear responsibility for assessing and documenting temperature
abuse.
This clause applies even where the company is not the final recipient.
Regulatory Reference: 21 CFR part 1 subpart O (1.908d)
6.5.5
Contracts with carriers
shall specify that the
carrier is responsible for
the following sanitary
activities where agreed to
in writing with shipper.
Sanitary condition of
vehicles and
transportation
equipment
Following shipper’s
sanitary specifications
Recording compliance
with operating
temperature where
critical to food safety
Where formally agreed upon with shipper, the U.S. carrier is responsible
for the following sanitary activities.
Ensuring vehicles and activities meet shipper's sanitary
specifications
Documenting compliance with specified operating temperature
where temperature controlled product is shipped (e.g., data
loggers or recording the temperature during loading and
unloading)
Pre-cooling of the refrigeration unit where specified by the
shipper
Identifying previous cargo of bulk transport to shipper upon
request
Disclosing the most recent cleaning of the vehicle to shipper upon
request
Establish and implement cleaning, sanitizing and inspection
procedures for ensuring vehicles and transportation equipment
are maintained in sanitary conditions
Regulatory Reference: 21 CFR part 1 subpart O (1.908e)
6.5.6
Contracts with carriers
shall specify that the
carrier implements a
training program for all
personnel engaged in
transportation activities,
which covers
Awareness of potential
food safety problems
that may occur during
food transportation
Basic sanitary
transportation
practices to address
those potential
problems
Responsibilities of the
carrier
The company shall document assurance that U.S. carriers implement
adequate training programs required by the Sanitary Transportation rule
in carrier contracts.
Training requirements defined in the Sanitary Transportation rule
include the following where the U.S. carrier is responsible for the sanitary
conditions of transportation operations.
Provide adequate training to all personnel engaged in
transportation regarding the awareness of potential food safety
problems that may occur during food transportation, basic
sanitary transportation practices to address those potential
problems, and the responsibilities of the carrier.
Provide training upon hire and periodically thereafter.
Maintain training records, which include: the date of the training,
the type of training, and the person(s) trained.
Regulatory Reference: 21 CFR part 1 subpart O (1.910)
6.5.7
The company shall keep all
records related to U.S.
transportation operations
and transportation service
contracts as original or
electronic records for a
minimum of 12 months
beyond termination of the
activity or contract. Offsite
Shippers shall retain records that they provide to carriers documenting
transportation specifications and operating temperatures as well as
carrier agreements for 12 months beyond termination of the agreement
with the carrier.
Carriers shall retain records of implemented procedures for 12 months
beyond expiration of transportation agreements and records of training
for 12 months beyond when the individual no longer performs the
activity.

16
records shall be retrievable
within 24 hours.
Any person providing services for compliance with the Sanitary
Transportation rule shall maintain records of activities and agreements
for 12 months beyond termination of the transportation agreement.
Electronic records are defined as onsite.
Regulatory Reference: 21 CFR part 1 subpart O (1.912)
17
Appendix 1
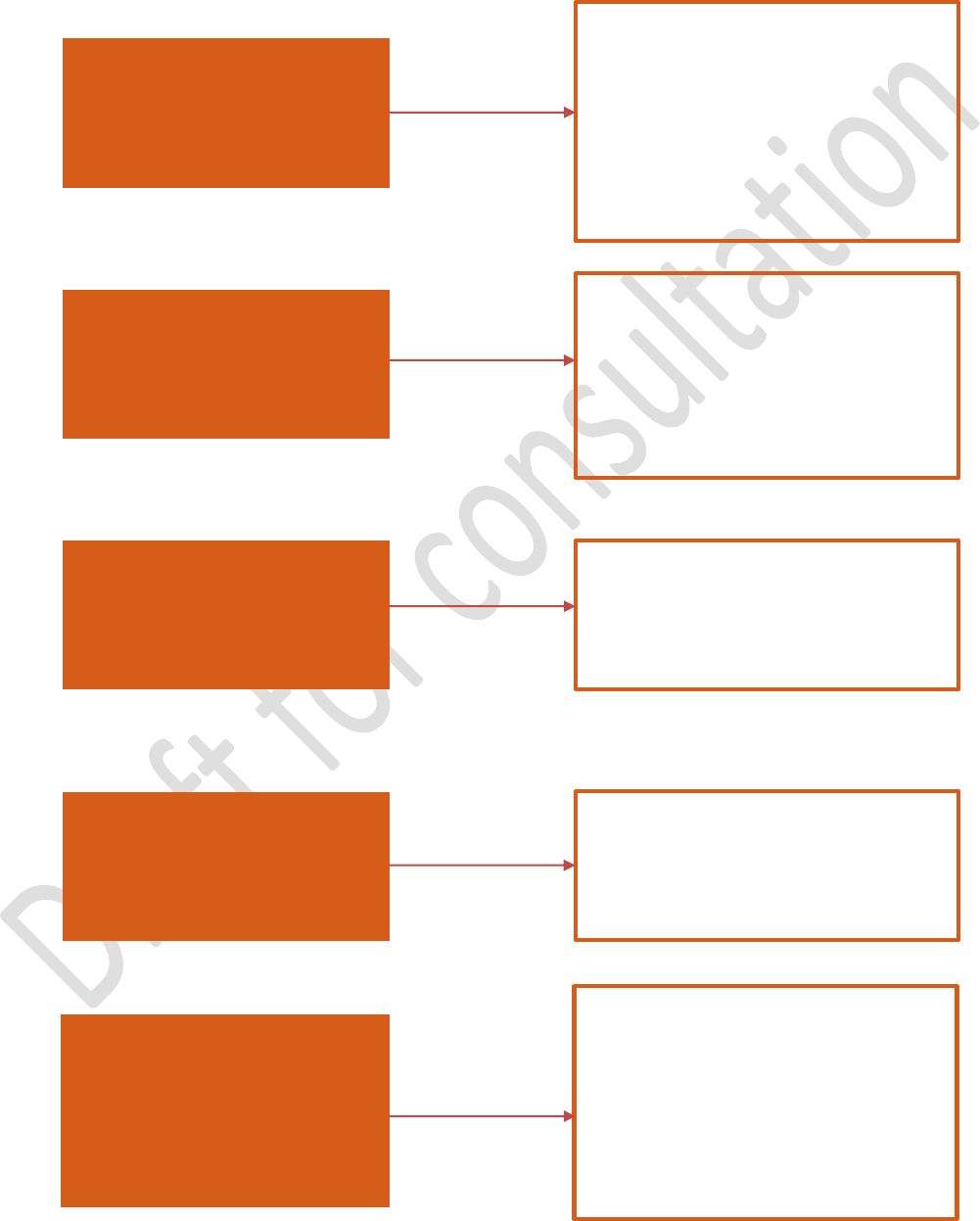
Correlation of some agent and broker activities with FSMA legislation
I trade and/or take title of
domestic (U.S. grown or
manufactured) human or
animal food products for sale in
the U.S.
I may be requested by my customer to
document and perform supplier
verification activities (21 CFR part 117
subpart G or 21 CFR part 507 subpart E)
where my customer (i.e., receiving facility)
has identified a hazard with a supply-
chain-applied control. The receiving
facility is responsible for review of my
verification.
I trade and/ or take title of foreign
supplied (non-U.S. grown or
manufactured) human or animal
food products for sale in the U.S.
I receive human or animal food in
the U.S. following transportation by
motor or road vehicle for food to be
sold within the U.S.
I arrange shipment of human or
animal food within the U.S. by one
or more carriers for food to be sold
within the U.S.
I take physical possession of human
or animal food products for the
purpose of manufacturing,
processing, packing or holding and
am registered as a food facility with
the U.S. FDA.
I must comply with 21 CFR part 1
subpart L:
Foreign Supplier Verification
Programs for Importers of Food for
Humans and Animals
(FSVP) and ensure
foreign suppliers I use are compliant with
FD&C sections 418, 419, 402, and 403(w)
unless excepted.
I must comply with 21 CFR part 1 subpart
O:
Sanitary Transportation of Human and
Animal Food
(ST) unless excepted.
I must comply with 21 CFR part 1 subpart
O:
Sanitary Transportation of Human and
Animal Food
(ST) unless excepted.
I must comply with 21 CFR part 117:
Preventive Controls for Human Food
(or
21 CFR part 507 for animal food) and 21
CFR part 121:
Mitigation Strategies to
Protect Food Against Intentional
Adulteration
unless excepted. I am
responsible for reporting an adulterated or
misbranded food.

18
Glossary
Carrier
A person or entity that physically moves food by rail or motor vehicle in commerce within the United
States. The term carrier does not include any person who transports food while operating as a parcel
delivery service.
Entity
A legal business.
Environmental Pathogen
A pathogen of the manufacturing, processing, packing, or holding environment, which can contaminate
food and cause foodborne illness if consumed. An example of an environmental pathogen common in wet
environments is
Listeria monocytogenes
. An example of an environmental pathogen common in dry
environments is
Salmonella
.
Food Defense
Activities, which protect food from intentional adulteration.
Foreign Supplier
A supplier that produces, processes, or manufactures food, which is exported to the United States.
Hazard requiring a control
A
known or reasonably foreseeable hazard
(see definition) requiring one or more controls to prevent,
significantly minimize or eliminate the hazard in the food as determined by a hazard analysis.
Holding
All activities related to the storage of food. Examples of holding activities include the following provided
that the activity does not process or transform the product: storage, drying, blending, fumigation, and
handling unexposed product. Holding facilities may include (but are not limited to) bulk silos, cold storage
facilities, grain elevators, liquid tanks, shipping containers, and warehouses.
Known or Reasonably Foreseeable Hazard
A biological, chemical (including radiological) or physical hazard associated with a food or the process/
facility of production.
Loader
A person or entity that loads food onto a motor or rail vehicle during transportation operations.
Mitigation Strategy
A risk-based measure determined from a vulnerability assessment, which is applied at a point in the
supply chain or at a process step to prevent or minimize a threat intending to cause wide scale public
health harm.
Qualified Auditor
An individual independent of the supplier who has appropriate education, technical expertise and
experience in auditing principles and food safety management systems to assess food suppliers for
compliance with the requirements of this Standard and regulatory compliance under the U.S. Food, Drug
and Cosmetic Act. Qualified auditors may be registered auditors with GFSI benchmarked schemes,

19
government inspectors or appropriately trained and experienced second-party auditors provided they
meet all expectations of this definition.
Qualified Individual
An individual who is responsible for conducting and/ or overseeing all activities of an agent or broker’s
food safety plan, foreign supplier verification program or other compliance program required by
legislation under the FSMA. The individual must have received appropriate education, training, and
experience to conduct such activities, which includes completion of the applicable FSMA training program
(e.g., FSPCA Preventive Controls or FSVP training courses).
Receiver
A person or entity who receives food at a point in the United States after transportation, whether or not
they represent the final point of receipt for the food.
Shipper
A person or entity (e.g., the manufacturer or a freight broker) who arranges for the transportation of food
in the United States by a carrier or multiple carriers sequentially.
Transportation Operations
All activities associated with transporting food, which may affect the sanitary condition of the food. This
includes but is not limited to cleaning, inspection, maintenance, loading and unloading, and operation of
vehicles and transportation equipment. Transportation operations do not include activities associated
with the transportation of food completely enclosed by a container except for food requiring temperature
control for safety, compressed food gases, food contact substances, human food byproducts transported for
use as animal food without further processing, or live food animals except molluscan shellfish.
Vulnerability Assessment
An evaluation of vulnerabilities within the supply chain or food production process, which can be
exploited to intentionally contaminate a product and cause wide spread public health harm. The
assessment must include an evaluation of the degree of physical access to product, the ability of an
attacker to successfully contaminate product and the severity and magnitude of harm in the event of
successful contamination.

20
FSMA References
Legislation
Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human
Food
Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Food for
Animals
Foreign Supplier Verification Programs for Importers of Food for Humans and Animals
Mitigation Strategies to Protect Food Against Intentional Adulteration
Sanitary Transportation of Human and Animal Food
Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption
Guidance
Food Facility Registration User Guide: Step-by-Step Instructions
Questions and Answers Regarding Food Facility Registration (Seventh Edition): Guidance for Industry
FSMA Rules & Guidance for Industry (a list of all FSMA related guidance documents)
Training
Food Safety Preventive Controls Alliance (FSPCA) Foreign Supplier Verification Program Training
Food Safety Preventive Controls Alliance (FSPCA) Intentional Adulteration Training
Food Safety Preventive Controls Alliance (FSPCA) Preventive Controls for Human Food Training
Food Safety Preventive Controls Alliance (FSPCA) Preventive Controls for Animal Food Training
Produce Safety Alliance (PSA) Training
