
REACH requirements for component suppliers and
equipment manufacturers
October 2008

REACH requirements for component suppliers and equipment manufacturers
Copyright
Copyright ENVIRON October 2008. All rights reserved.
This Guide may be freely reproduced in its original form. Electronic copies are available
from www.BOMcheck.net
.
Author
Dr Aidan Turnbull
Head of WEEE, RoHS and EcoDesign
ENVIRON
Box House, Box
Wiltshire SN13 8AA
UK
Tel: +44 1225 748420
Fax: +44 1225 748421
e-mail: aturnbull@uk.environcorp.com
ENVIRON welcomes all constructive feedback and suggestions for new content in future
editions.
Acknowledgements
We wish to recognize the efforts of Dr. Freimut Schröder, Siemens Healthcare, who had the
vision and idea to create a commercial tool to manage supplier material information in a
simple and cost-effective way, and Dr. Aidan Turnbull, ENVIRON, who created BOMcheck to
realize this vision. We would like to thank the members of the BOMcheck Steering Group
who provided valuable feedback during the writing of this Guide:
Siemens
Philips
GE
Texas Instruments
Agfa
We would also like to thank Apple and Abbott for their valuable contributions.
Disclaimer
This Guide comprises ENVIRON’s opinion concerning how to comply with and respond to
the REACH Regulation. This opinion is based on the published legislation and guidance,
and extensive research and consultation but is not legally binding. A binding interpretation
of Community legislation is the exclusive competence of the European Court of Justice.
Whilst great care has been taken in the compilation and preparation of this Guide, use of
the information in this Guide is entirely at the risk of the user. ENVIRON can not accept
responsibility or liability for loss or damage occasioned by any person or property acting or
refraining from action as a result of any material in this publication.

REACH requirements for component suppliers and equipment manufacturers
Executive Summary
REACH introduces new requirements on EU component suppliers to provide substance
declarations (Article 33) and comply with substance restrictions (Article 67) when they
supply their articles (e.g. components and sub-assemblies) to the next manufacturer in the
supply chain. REACH is fundamentally different to RoHS where there is no legal
obligation on EU suppliers to provide information on the RoHS compliance status of their
components and sub-assemblies.
Starting from October 2008 when the first Candidate List was published, Article 33 (1) of
the REACH Regulation places a legal obligation on all EU suppliers to inform their
manufacturing customers whether the components or assemblies they supply contain any
of the REACH Candidate List substances in concentrations > 0.1% w/w. For all
components or assemblies which exceed this concentration, the supplier has a legal
obligation to provide information on safe use.
From this Candidate List list, by June 2009 ECHA will select the prority substances that will
become the first to be considered for Authorisation under REACH. Unless covered by an
exemption, companies who wish to continue using these priority substances in the EU will
need to apply for an authorisation. The cost and inconvenience of the authorisation
conditions are likely to deter all but the most essential or profitable applications.
From June 2009, Article 67 of REACH places a legal obligation on all EU suppliers to comply
with the substance restrictions listed in Annex XVII when they supply components or sub-
assemblies to manufacturing customers
.
Manufacturers should insist that their suppliers comply with these legal obligations as this
information enables the manufacturers to make early strategic decisions on whether to
design-out components which contain the Candidate List substances. Manufacturers also
need this information to assist them to meet their legal obligations to comply with Article 67
substance restrictions and to provide information on Candidate List substances under
REACH Article 33 when they supply products to their customers.
The BOMcheck substances declarations web database has been developed to help
manufacturers and suppliers manage this information up the supply chain, keep up-to-date
with new substances as they are added to the Candidate List and new substance
restrictions added to Annex XVII. BOMcheck (www.BOMcheck.net
) is an industry-wide
initiative which has been developed by Siemens and ENVIRON and is led by the European
trade association COCIR (www.cocir.org
). COCIR membership includes Siemens, Philips,
GE, Agfa, Hitachi, Toshiba, IBM, Intel and Canon. The web database covers REACH,
RoHS and other restricted substances legislation. Membership is free for manufacturers
and costs 300 Euros per year for suppliers.

REACH requirements for component suppliers and equipment manufacturers
Contents
1 Introduction...................................................................................................................1
2 REACH obligations on chemicals manufacturers and importers....................................2
3 How the REACH definition of an article applies to components and equipment ............4
4 As an article manufacturer or importer, do you have any obligations to register and
investigate chemicals?..................................................................................................6
4.1 Importing articles into the EU accompanied by substances or preparations......................6
4.2 Articles which act as containers for intentional release of substances or preparations......6
4.3 Articles which act as a carrier material for intentional release of substances or
preparations ......................................................................................................................7
4.4 Other intentional release of substances or preparations from articles ............................... 7
5 Critical component supplier REACH risk assessment ...................................................8
6 Authorisation of substances of very high concern (SVHC) ..........................................10
7 Candidate List substances for authorisation................................................................12
8 Requirement for component suppliers to provide substance declarations to
manufacturers.............................................................................................................14
8.1 Supplier substance declarations......................................................................................15
8.2 Information on safe use...................................................................................................16
8.3 Ongoing supplier compliance requirements from October 2008......................................17
9 Substance disclosure requirements for imported assemblies......................................17
10 Requirement for manufacturers to provide substance declarations to consumers on
request........................................................................................................................19
10.1 Substance declarations and information on safe use ...................................................... 19
10.2 NGO action to promote consumer awareness of the Candidate List ............................... 19
11 Additional requirements for Candidate List substances in articles...............................20
11.1 Article 7 (2) notification of substances in articles............................................................. 20
11.2 Article 66 notification of authorised substances in articles...............................................21
12 Substance restrictions enforced by REACH ................................................................21
12.1 Practical issues with the current Marketing and Use Directive ........................................22

REACH requirements for component suppliers and equipment manufacturers
12.2 Improved regulation of substance restrictions under REACH Article 67.......................... 23
12.3 REACH restricted substances that may be found in EEE................................................ 25
13 BOMcheck web database to enable component suppliers to comply with REACH
requirements...............................................................................................................27
13.1 How is industry leading this initiative?............................................................................. 27
13.2 What are the benefits and costs to suppliers?................................................................. 29
13.3 What are the benefits and costs to manufacturers? ........................................................33
14 Managing RoHS compliance.......................................................................................38
14.1 How does BOMcheck manage changes in RoHS exemptions?....................................... 38
14.2 How does BOMcheck manage compliance with RoHS restrictions in China, Korea,
Japan? ............................................................................................................................38
14.3 What are the new substances that may be added to RoHS?...........................................39
15 About ENVIRON .........................................................................................................40
16 Further information......................................................................................................41
ANNEX 1: Key REACH Articles for equipment manufacturers / importers .......................42
Article 7: Registration and notification of substances in articles................................................ 42
Article 33: Duty to communicate information on substances in articles.....................................43
Article 67: Restrictions on the manufacturing, placing on the market and use of certain .........
dangerous substances, preparations and articles .................................................... 43
ANNEX 2: Safety Data Sheet or similar safety information requirements for supply of ........
substances and preparations purchased in the EU .......................................44
ANNEX 3: BOMcheck letter for manufacturers to send to suppliers.................................46
ANNEX 4: BOMcheck letter for suppliers to send to manufacturers.................................48

REACH requirements for component suppliers and equipment manufacturers
1 of 50
1 Introduction
The REACH Regulation became law throughout the EU on 1 June 2007 and has a phased
program of implementation over 11 years. Described as 'the most ambitious chemicals
legislation in the world', REACH will apply to about 30,000 chemical substances which are
currently in use across Europe. REACH is an EC Regulation and is directly applicable
throughout all EU Member States. It does not require transposition into national laws of
Member States, but does require each Member State to set up a system of controls and
penalties for non-compliance. The REACH Regulation has also been adopted in the EEA
EFTA States (Iceland, Liechtenstein, and Norway). REACH applies in Iceland,
Liechtenstein and Norway in the same way as in the EU Member States.
REACH stands for the Registration, Evaluation, Authorisation and Restriction of
Chemicals. The Registration and Evaluation elements apply to companies that import or
manufacture more than 1 tonne per year of chemical substances in the EU, and are
summarised in section 2. However, the Authorisation and Restriction elements affect all
companies which manufacture or import into the EU, including component suppliers and
equipment manufacturers. Components and equipment are known as “articles” under
REACH. The definition of an “article” is discussed in detail in section 3.
The first step for component suppliers and equipment manufacturers is to check that none
of the articles they manufacture or import would cause them to be regarded as a chemicals
supplier under REACH. In other words, they need to check that none of their articles
would require them to carry out registration and evaluation of chemical substances. This is
addressed in section 4 of this Guide. It is also important to carry out a critical supplier
REACH risk assessment. This identifies whether the availability of components from
critical suppliers is at risk because chemical substances are not registered for that use
under REACH by an upstream supplier. This is discussed in section 5 of the Guide, which
includes a standard letter to send to chemicals suppliers.
The Authorisation element is directly relevant to component suppliers and equipment
manufacturers as this will place limits on the use of an increasing number of chemical
substances. Under REACH, companies will need to apply for authorisation to continue to
use certain chemical substances of very high concern (SVHC). The first Candidate List
containing the first 15 SVHC substances was published in October 2008 and the
authorisation process will start from 2009. More chemical substances will be added to the
Candidate List periodically by Member States, the European Commission and ECHA.
Starting from October 2008 when the first Candidate List was published, REACH places a
legal obligation on all EU component suppliers to inform manufacturers further down the
supply chain whether the articles they supply to the manufacturer contain any of the
Candidate List substances in concentrations > 0.1% weight by weight (w/w) of the
component. This enables the manufacturers to make early strategic decisions on whether
to design-out components which contain these chemical substances. In turn, from October
2008 REACH places a legal obligation on manufacturers and importers to disclose to
consumers on request whether their finished equipment contain > 0.1% w/w of any
Candidate List substance. This allows consumers to choose whether to buy equipment
which contain these substances of very high concern.
REACH is fundamentally different to RoHS in this regard. Under RoHS, the legal
responsibility for ensuring compliance with the substance restrictions rests solely with the

REACH requirements for component suppliers and equipment manufacturers
2 of 50
OEM manufacturer or importer who puts the finished branded equipment
1
on the market in
the EU. There is no legal obligation on EU suppliers to provide information on the RoHS
compliance status of their components and sub-assemblies. As a result, OEM
manufacturers and importers incurred considerable costs to gather the necessary
compliance information from their suppliers. For example, some contract manufacturers
offer to gather this RoHS compliance information from suppliers as an additional business
service to their OEM clients.
In contrast, REACH places a legal obligation on all EU suppliers to provide substance
declaration information when they supply their articles (e.g. components and sub-
assemblies) to the next manufacturer in the supply chain. For example, this includes
contract manufacturers when they supply equipment to OEM clients, drawing on
information which component suppliers are required to disclose to the contract
manufacturer.
Gathering and managing these substance declarations through the supply chain
represents a considerable challenge for manufacturers and suppliers. Suppliers need
guidance on the Candidate List substances to help them make their declarations to
manufacturers. Manufacturers and suppliers need a system to help them manage this
information up the supply chain to the consumer, and keep up-to-date with new SVHC
substances as they are added to the Candidate List. Manufacturers need to implement
these systems early so that they can plan for any required product design changes and
minimise any disruption to sales and maintenance of their equipment. The BOMcheck
substances declarations web database has been developed specifically to enable
management of this information through the supply chain.
The Restriction element is enacted by Article 67 of REACH. From June 2009 this brings
into force the list of substance restrictions in Annex XVII of REACH, and replaces the
substance restrictions which are currently contained in the Marketing and Use Directive
2
.
In addition to substances which are already restricted under the RoHS Directive, this list
includes a further 18 substance restrictions which are potentially relevant to electrical and
electronic equipment, which are discussed in section 7 of this Guide. All companies that
manufacture or import components or equipment into the EU are required to comply with
these substance restrictions. As above, suppliers need guidance on these substance
restrictions to help them declare their compliance to the next manufacturer in the supply
chain. BOMcheck provides this solution.
2 REACH obligations on chemicals manufacturers and importers
REACH requires chemicals suppliers to submit a registration dossier to the new
European Chemicals Agency (ECHA) in Helsinki for any chemical substance that they
manufacture in the EU or import into the EU in quantities greater than 1 tonne per year per
legal entity. As part of the registration process, the chemicals suppliers are required to
investigate the environmental and health and safety aspects of the chemical substance
through a comprehensive program of data collection, testing and assessment. The
chemicals suppliers are required to provide safety information down the supply chain so
that the risks arising from the use of the chemical substances can be managed properly.
1
The RoHS substance restrictions apply to finished products which fall under Categories 1 to 7 and 10 of Annex I of the WEEE
Directive
2
76/769/EC

REACH requirements for component suppliers and equipment manufacturers
3 of 50
ECHA will then evaluate whether further investigation of the substance is needed and
what further information needs to be provided by industry for that purpose. This
information may lead to further risk management actions under the restrictions or
authorisations procedure.
REACH Article 3 (1) provides the following definition of a substance
“Substance means a chemical element and its compounds in the natural state or
obtained by any manufacturing process, including any additive necessary to
preserve its stability and any impurity deriving from the process used, but excluding
any solvent which may be separated without affecting the stability of the substance
or changing its composition”
REACH Article 3 (2) provides the following definition of a preparation
“Preparation means a mixture or solution composed of two or more substances”
For existing substances (i.e. in simplified terms these are substances that are already in
use in the EU as per Article 28 (3)), there is an option to pre-register and take advantage
of a phase-in program for registration. The phase-in program prioritizes substances based
on higher volumes and certain substances that have irreversible health effects or may
cause long-term adverse impacts to the aquatic environment.
Table 1: Timescales for pre-registration and registration of phase-in substances
Activity Timescale
Pre-registration of phase-in substances 1 June 2008 – 1 December 2008
Registration of phase-in substances which
are either:
• CMR substances > 1 tonne per year, or
• R50/53 substances > 100 tonnes per
year, or
• Any substance > 1,000 tonnes per year
1 June 2008 – 30 November 2010
Registration of any phase-in substance >
100 tonnes per year
1 June 2008 – 31 May 2013
Registration of any phase-in substance > 1
tonne per year
1 June 2008 – 31 May 2018
If a phase-in substance is not pre-registered, then no transition periods for registration will
be allowed and the substance will have to be registered before the supplier can continue
manufacturing, importing or putting more than 1 tonne per year of the substance on the
market. From 1 June 2008, new substances (e.g. a new formulation) and non-phase-in
substances must be registered before they are placed on the market in quantities greater
than 1 tonne per year.

REACH requirements for component suppliers and equipment manufacturers
4 of 50
There are additional requirements for substances that the chemicals supplier imports into
or manufactures in the EU in quantities greater than 10 tonnes per year. In this case, the
chemicals supplier’s registration dossier must include a chemicals safety assessment
(CSA) which will be documented in the chemical safety report (CSR), in accordance with
Article 10 and Article 14. If the substance is considered as “dangerous” according to the
Dangerous Substances Directive or as PBT (persistent, bioaccumulative and toxic) or
vPvB (very persistent and very bioaccumulative) according to Annex XIII of REACH, then
the CSA must be completed with an exposure scenario (ES). The ES describes the
conditions under which the risks to human health and environment are “adequately
controlled”. In particular, it describes how the substance (on its own, in preparations or in
articles) is manufactured and used throughout the lifecycle, and provides recommended
risk management measures (e.g. use of personal protective equipment, need for local
exhaust ventilation) and operational conditions (e.g. duration and frequency of use), so as
to ensure safe use of the substance. Downstream users have the right to communicate
their uses of the substance to their suppliers, who in turn are required to consider these
uses in the ES.
ENVIRON provides a range of detailed services to enable chemicals suppliers to meet
their REACH obligations. Further details about ENVIRON’s REACH services for chemical
suppliers are available at www.environcorp.com/reach
.
3 How the REACH definition of an article applies to components and
equipment
REACH provides the following definition of an “article” in Article 3 (3):
“an object which during production is given a special shape, surface or design
which determines its function to a greater degree than its chemical composition”
An easy example is a polystyrene cup. Although it is pure polystyrene, its form (a cup
shape) means that it is classed as an article and not a substance or preparation. Other
examples include board-level components, a circuit board itself, a screw, a bolt, a motor, a
battery, a power supply unit, packaging etc. Note that packaging is treated as a separate
article in its own right.
“Producer of an article” is defined in Article 3 (4) as
“any natural or legal person who makes or assembles an article within the
Community”
“Supplier of an article” is defined in Article 3 (33) as
“any producer or importer of an article, distributor or other actor in the supply chain
placing an article on the market”
“Placing on the market” is defined in Article 3 (12) as
“supplying or making available, whether in return for payment or free of charge, to a
third party. Import shall be deemed to be placing on the market.”
“Recipient of an article” is defined in Article 3 (35) as
“an industrial or professional user, or a distributor, being supplied with an article but
does not include consumers”
REACH requirements for component suppliers and equipment manufacturers
5 of 50
The supply chain can involve several tiers of suppliers who each place articles on the
market for assembly into more complicated articles by the next producer in the chain. For
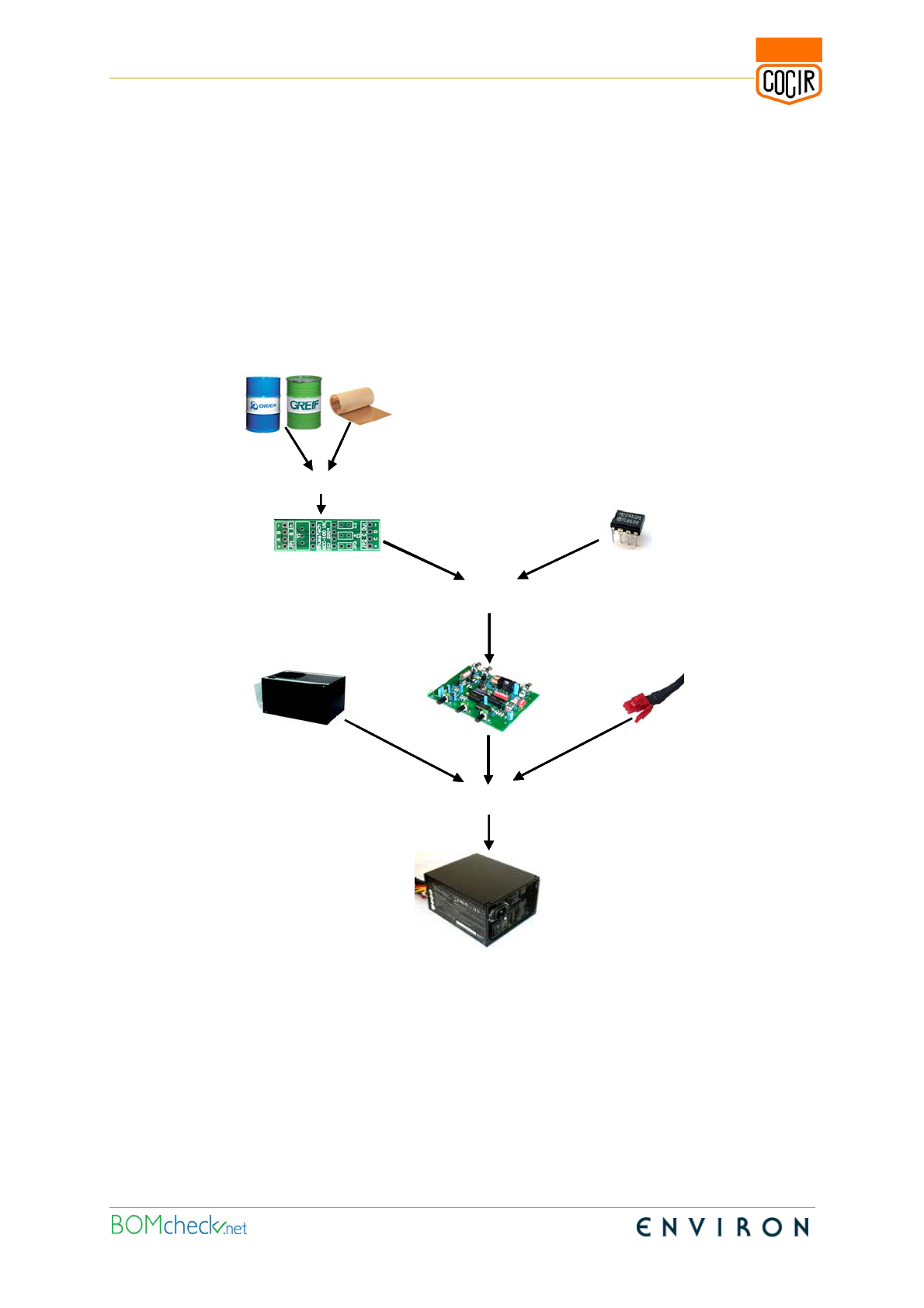
example, take the manufacture of a power supply unit which is carried out by a contract
manufacturer within the EU, starting from an FR4 circuit board which is manufactured from
an epoxy resin, Figure 1. The circuit board manufacturer forms the circuit board from the
epoxy resin (a preparation) and copper foil to create an article which it supplies to a circuit
board assembler. The circuit board assembler purchases commodity components
(articles) from suppliers who have imported the components into the EU. The circuit board
assembler populates the board to create another article (the assembled board) and
supplies this to the contract manufacturer. The contract manufacturer then assembles the
populated circuit board into the housing (another article) together with cables (more
articles) to form the power supply unit (another article).
Board manufacturer
PCB assembler
Board-level
component supplier
Cable supplierHousing manufacturer
Assembled power supply unit
Contract manufacturer
Substances and preparations
Article Article
ArticleArticle
Article
Article
Board manufacturer
PCB assembler
Board-level
component supplier
Cable supplierHousing manufacturer
Assembled power supply unit
Contract manufacturer
Substances and preparations
Article Article
ArticleArticle
Article
Article
Figure 1: Articles placed on the market by producers and suppliers for the manufacture of a power
supply unit carried out by a contract manufacturer in the EU
In the example in Figure 1, the board manufacturer, PCB assembler, housing
manufacturer and contract manufacturer are all producers that place articles on the
market. In this example, the component supplier and cable supplier are importers of
articles which are manufactured outside the EU. The component supplier and cable
supplier have the same REACH compliance requirements as the board manufacturer, PCB
assembler and housing manufacturer who manufacture articles within the EU.

REACH requirements for component suppliers and equipment manufacturers
6 of 50
4 As an article manufacturer or importer, do you have any obligations
to register and investigate chemicals?
This set of four questions checks whether there are any circumstances under which a
component supplier or equipment manufacturer would be regarded as importing
substances or preparations into the EU. In other words, do the articles that you import or
manufacture in the EU mean that the REACH Regulation treats you like a chemical
supplier. If you can answer “No” to all of these questions then you do not have any
obligations to register or investigate chemicals for REACH. In particular, you do not have
to submit a registration dossier to the new European Chemicals Agency (ECHA) in
Helsinki under Article 6 or Article 7 (1). If you answer “Yes” to these questions, then you
need to contact ENVIRON for further advice.
4.1 Importing articles into the EU accompanied by substances or preparations
Question Answer
Do you import components or equipment into the EU? If so, is any
equipment or component accompanied by substances or preparations?
For example, do you import equipment which is packaged together with
reagents, lubricants, gels?
Yes / No
If the answer to the above question is yes, do you import more than 1
tonne per year of this substance into the EU?
Yes / No
If the answer to both of these questions is yes, then contact ENVIRON as soon as possible
for assistance in understanding and complying with obligations for registration of
substances under REACH Article 6. The deadline for pre-registration of substances for
REACH is 1 December 2008.
4.2 Articles which act as containers for release of substances or preparations
Question Answer
Does any component or equipment that you manufacture or import into
the EU act as a container for release of substances or preparations when
it is put on the market? For example, a printer cartridge containing inks?
In this case, the main function of the cartridge is to control the release of
ink.
Yes / No
If yes, do you put more than 1 tonne per year of this substance on the
market in the EU?
Yes / No
If the answer to both these questions is yes, then contact ENVIRON as soon as possible
for assistance in understanding and complying with obligations for obligations for
registration under REACH Article 6. The deadline for pre-registration of substances for
REACH is 1 December 2008.

REACH requirements for component suppliers and equipment manufacturers
7 of 50
Explanatory Note
Manufacturers and importers of an article which acts as a container for release of
substances of preparations are treated like a chemical supplier. In other words, a
manufacturer or importer that supplies a pen is treated like a chemical supplier that
supplies the ink.
4.3 Articles which act as a carrier material for release of substances or
preparations
Answer
Does any component or equipment that you manufacture or import into
the EU act as a carrier material for release of substances or preparations
when it is put on the market? For example, a cloth impregnated with
polish? In this case the main function of the cloth is to release polish.
Yes / No
If yes, do you put more than 1 tonne per year of this substance on the
market in the EU?
Yes / No
If yes, then contact ENVIRON as soon as possible for assistance in understanding and
complying with obligations for obligations for registration under REACH Article 6. The
deadline for pre-registration of substances for REACH is 1 December 2008.
Explanatory Notes
Manufacturers and importers of an article which acts as a carrier material for release of
substances or preparations are treated like a chemical supplier. In other words, a
manufacturer or importer that supplies a cloth impregnated with polish is treated like a
chemical supplier that supplies the polish.
4.4 Other intentional release of substances or preparations from articles
Answer
Does any component or equipment that you manufacture or import into
the EU contain any other substances or preparations which are intended
to be released during normal and reasonably forseeable conditions of
use? For example, release of perfume from a scented eraser?
Yes / No
If yes, do your articles release more than 1 tonne per year of this
substance in the EU?
Yes / No
If yes, then contact ENVIRON as soon as possible for assistance in understanding and
complying with obligations for registration under REACH Article 7 (1). The deadline for
pre-registration of substances for REACH is 1 December 2008.

REACH requirements for component suppliers and equipment manufacturers
8 of 50
Explanatory Notes
In practice, there are very few circumstances where an article contains a substance which
is intended to be released, but where the article is not acting as a container of the
substances or a carrier material for a substance. In the context of Article 7 (1), an
intended release is deliberately planned and has a specific function for the article, but it is
frequently not the main function of the article. An often quoted example of an article which
falls under Article 7 (1) is the release of perfume from a scented eraser.
A release is not considered to be an intended release in the following cases:
• A release from the article occurs during use or maintenance (which is carried out to
improve product quality or safety) and the release does not contribute to the function of
the article. For example, releases from washing clothes where remnants of different
chemicals used in clothing manufacture (dyes, softeners, starch etc) are removed over
several washing cycles.
• A release of a substance which is an unavoidable side-effect of the functioning of the
article. Without the release the article would not work, but the release is not directly
intended. For example, wear and tear of materials under high friction, such as break
linings, tyres etc.
• A release which is an unavoidable consequence of the product function, or which is
formed during a chemical reaction of any kind. For example, ozone released from
photocopier machines. Also releases of substances from chemical reactions caused
by accidents or product malfunctions, for example combustion products from an article
catching fire.
• A release which is incidental, arising from an accident or inappropriate use. For
example, a thermometer which drops and breaks. This also includes any form of
misuse and inappropriate use which is not in accordance with the manufacturers
instructions for use, or the functionality of the product – even if it could have been
anticipated.
5 Critical component supplier REACH risk assessment
Manufacturers should consider carrying out a REACH risk assessment of their critical
component suppliers. This identifies whether the availability of components from critical
suppliers is at risk because substances are not registered for that use under REACH by an
upstream supplier in the EU. The risk that failure to comply with REACH requirements
may threaten the continued availability of components from critical suppliers will depend on
a number of factors including:
• The size and level of REACH awareness of the supplier. Small suppliers are less likely
to be aware of the need to check with their upstream suppliers in the EU whether
substances that they use in the manufacturing process or for supply with the
component, have been pre-registered by 1 December 2008 and will be registered for
that use.
• The component supplier is using specialist substances during the manufacturing
process or for supply with the component. In this case, it is important for the supplier
to check with their upstream suppliers in the EU whether these specialist substances
have been pre-registered by 1 December 2008 and will be registered for that use.

REACH requirements for component suppliers and equipment manufacturers
9 of 50
• The component supplier is using substances in an unusual way. As above, it is
important for the supplier to check with their upstream suppliers in the EU whether this
unusual use of the substance will included in the registration dossier.
For a critical component supplier where continued availability of components may be at
risk, the manufacturer should require the component supplier to write to their upstream
chemical supplier to obtain confirmation that:
A. the upstream chemical supplier intends to pre-register the substances by 1 December
2008, and carry out full registration by the relevant deadline date
B. the upstream chemical supplier will include the critical suppliers use of the substance in
their registration dossier
For example, a small supplier in the EU which is manufacturing bespoke high voltage
transformers for an OEM and is using a specialist chemical preparation to coat the coils for
electrical isolation. The OEM would identify the continued availability of transformers from
this strategically important supplier as being at risk in case the upstream chemical supplier
does not:
• Pre-register the substances used in the chemical preparation by 1 December 2008;
• Register the substances for use in an electrical isolation coating.
The OEM should require the transformer supplier to write to their upstream chemical
supplier to gain confirmation on these issues as soon as possible, so that the OEM can
take appropriate action. ENVIRON has developed a standard letter that the critical
component supplier can adapt and send to their upstream chemical supplier, Figure 3.
[Supplier name and address]
[Date]
Dear [Supplier]
To ensure continued compliance with the EC REACH Regulation, please can
you confirm the following details so that we can continue to buy
substances and preparations from you.
Delete as
applicable
We confirm that we will pre-register the following
substance/ all substances in the following preparation
[delete as applicable] by 1 December 2008
[Provide the name of substance or preparation here]
Yes / No
We confirm that we will register the above substance/ all
substances in the above preparation [delete as applicable]
by the deadline dates specified in the REACH Regulation
Yes / No

REACH requirements for component suppliers and equipment manufacturers
10 of 50
We confirm that the registration dossier for the above
substance/ all substances in the above preparation [delete
as applicable] will include the following use [Provide a
brief description of your use of the substance or
preparation here]
Yes / No
Signature
Name
Position
On behalf of [Supplier name]
Date
Please returned a signed copy of this letter by XX/XX/2008.
If you are not in a position to answer these questions (e.g. because you
are not the first importer or manufacturer of the substance or
preparation in the EU) then please forward this letter to your supplier
and ask them to provide confirmation to you asap, as per the above
timescales, so that we can continue to buy from you.
Thank you for your cooperation.
Yours sincerely.
Figure 3: Standard letter for component suppliers and equipment manufacturers to send to their
chemicals suppliers
6 Authorisation of substances of very high concern (SVHC)
The Authorisation element of REACH is directly relevant to component suppliers and
equipment manufacturers as this will place limits on the use of an increasing number of
chemical substances. By June 2009 ECHA will select the prority substances from the
Candidate List (see section 7) that will become the first to be considered for Authorisation
under REACH. Substances that will be included in the Authorisation list cannot be
manufactured or imported into the EU from a specific date set by the Commission, except
if the companies have obtained an authorisation for their specific use(s). This
authorisation can be granted either because the risks are controlled or because the socio-
economic benefits outweigh the risks. The aim of the authorisation process is to ensure
that risks from these priority substances are properly controlled and that these substances
are progressively replaced by suitable alternative substances or technologies.
As noted by ECHA
3
, the substances which are included in the authorization process have
intrinsic hazardous properties of such concern that the Community needs to decide about
the adequacy of the control of the risks arising from their uses or whether the socio-
3
Guidance on Inclusion of Substances in Annex XIV, ECHA, August 2008

REACH requirements for component suppliers and equipment manufacturers
11 of 50
economic benefits outweigh the risks arising from the use of such substances. To this end
all manufacturers, importers and downstream users applying for authorisations are
required to analyse the availability of alternatives, consider their risks, and the technical
and economic feasibility of substitution (Article 55).
The process that ECHA follows to create the Candidate List of substances, from which the
priority list requiring authorization will be drawn, is set out in Article 59 and discussed in
section 7. The first Candidate List contains 15 SVHC substances and was published in
October 2008. More substances will be added to the Candidate List periodically by
Member States, the European Commission and ECHA.
Between January and April 2009, ECHA will publicly consult on the deadline date by which
applications for authorisation to use these priority substances must be received, and any
uses which should be permanently exempted from authorisation. The final details on
deadline dates for authorisation applications and any permanent exemptions for specific
uses will be agreed by the European Commission and recorded in Annex XIV of REACH.
Once the final details have been published in Annex XIV, companies who wish to continue
to use the substances (in a use not covered by a permanent exemption) will need to apply
for an authorisation for that use, regardless of the quantity of the substance used. The
authorisation process will start from late 2009 and all applications must be received by the
deadline dates specified in Annex XIV. Companies should, of course, check whether an
upstream user has already submitted an application that would cover their use. The
application must include an analysis of alternatives and where suitable alternatives are
available, timescales for substitution plans.
The European Commission will grant an authorisation if the applicant can demonstrate that
the risk from the use of the substance is adequately controlled. Each authorisation will be
subject to a time-limited review, which will be set on a case-by-case basis in accordance
with Article 61. Similar to exemptions under the RoHS Directive, authorisations may be
amended or withdrawn as a result of the review. In addition, authorisations can be
amended or withdrawn at any time when new information on possible substitutes becomes
available or the circumstances in the original authorisation have changed so as to affect
the risk to human health or the environment, or the social-economic impact.
As noted by the ENDS report
4
, the cost and inconvenience of the conditions expected to
be placed on authorized substances are likely to deter all but the most essential or
profitable applications.
Starting from October 2008 when the first Candidate List was published, REACH places a
legal obligation on all EU component suppliers to inform manufacturers further down the
supply chain whether their components contain any of the Candidate List substances in
concentrations > 0.1% weight by weight (w/w) of the component. This enables the
manufacturers to make early strategic decisions on whether to design-out components
which contain these substances. Most manufacturers will choose to phase out Candidate
List substances from their equipment for the following reasons:
• For uses not covered by a permanent exemption in Annex XIV, the continued
manufacturing of components in the EU which contain priority substances drawn from
the Candidate List will depend on whether an authorisation is issued by the European
Commission. There is no guarantee that the Commission will issue an authorisation.
4
ENDS Report 402, July 2008

REACH requirements for component suppliers and equipment manufacturers
12 of 50
Furthermore, each authorisation will be for a limited time period after which the
authorisation will be reviewed and could be removed. Continued use of these
components represents a business risk to the manufacturer that the components may
no longer be available in the EU.
• To avoid this business risk, most companies will choose to phase out components
containing the Candidate List substances where possible. As a result, companies that
continue to use components containing these substances will find that they become
more expensive and have less availability.
• Starting from October 2008 REACH places a legal obligation on manufacturers and
importers to disclose to consumers on request whether their finished equipment
contains > 0.1% w/w of any Candidate List substance. This allows consumers to
choose whether to buy equipment which contain these substances of very high
concern. As consumer awareness of these substances increases, this will bring
increasing pressure for manufacturers to phase out the use of these substances.
7 Candidate List substances for authorisation
The process that ECHA follows to create the Candidate List of substances (whose
continued use will require authorisation under REACH) is set out in Article 59. ECHA
published the first Candidate List of 15 substances in October 2008, Table 2. ECHA has
acknowledged that this first Candidate List would have a “high profile” and that the
timeframe for component suppliers and equipment manufacturers to react to the list was
very short.
Table 2 analyses this first Candidate List and identifies whether the substances are likely
to be found in electrical and electronic equipment.
The Candidate List will be updated regularly in response to applications to add new
substances to the list made by Member States and ECHA acting on behalf of the European
Commission. For example, the European Commission has already requested ECHA to
prepare applications (known as Annex XV dossiers) to add another 5 substances to the
Candidate List. The names of these 5 substances will be published in later in 2008 and
the substances are expected to be added to the Candidate List in early 2009.
In future, substances may be added to the Candidate List simply because they are already
identified in other items of legislation as being hazardous. For example, Annex 1 of the
Dangerous Substances Directive
6
lists about 800 substances which are already identified
as CMR (carcinogenic, mutagenic or toxic for reproduction) category 1 or 2 substances.
Article 59 specifically notes that when ECHA or a Member State prepares a dossier for a
substance to be included in the Candidate List, the dossier “may be limited, if appropriate,
to a reference to an entry in Annex 1 of the Dangerous Substances Directive”.
6
Directive 67/548/EEC

REACH requirements for component suppliers and equipment manufacturers
13 of 50
Table 2: The 15 substances of very high concern in the first Candidate List
Substance Likely to be found in electrical and electronic equipment?
Anthracene No. The only current commercial use of anthracene is in pyrotechnics
used for film and theatre productions as a component of black smoke.
Anthracene is also found in coal tar derivatives such as creosote used
for wood treatment.
MDA (4,4'-
Methylene
dianiline)
No. Less than 4,000 tonnes of MDA per year are used as a hardener
for epoxy resins, hardener in adhesives and intermediate in the
manufacture of polyurethane. However, in all cases the MDA is reacted
in a polymerisation process and so no free MDA is ever found in any
component of electrical and electronic equipment.
DBP (dibutyl
phthalate)
Yes. DBP is often used, in combination with other phthalates, in flexible
PVC. Typical phthalate content in PVC ranges from 30 to 45% w/w, of
which DBP is a major component at up to 15%. DBP is also used in
neoprene and nitrile rubber, PVA adhesives, nitrocellulose lacquers,
printing inks, sealants and coatings.
Cobalt
dichloride
Very unlikely. Used as a humidity indicator in hygrometers, barometers
and self-indicating silica gels and also as an absorbent agent for
ammonia gas (e.g. gas masks).
Diarsenic
pentoxide
Very unlikely. The nearest application to the electrical and electronics
industry is for the manufacturing of certain types of glass.
Diarsenic
trioxide
Very unlikely. Although diarsenic trioxide is used in the manufacturing
process for arsenide semiconductors, diarsenic trioxide is not found in
the manufactured semiconductor components. The nearest application
to the electrical and electronics industry is for the manufacturing of
certain types of glass.
Sodium
dichromate
(dehydrate
form)
No. Sodium dichromate (dehydrate form) is used in the metal finishing
industry for chrome plating and corrosion resistance (passivating and
anodising). However, sodium dichromate itself is not found in or on the
treated metal surfaces.
Musk xylene No. Musk xylene has been used since the early 1900s as a fragrance
ingredient in perfumes, soaps, detergents and cosmetics.
DEHP Yes. DEHP is widely used as a plasticiser in polymer products, mainly
PVC. In flexible PVC the typical phthalate content ranges from 30 to
45% w/w. DEHP is also used in other vinyl resins, cellulose ester
plastics, dielectric fluid in capacitors, adhesives, sealants, lacquers and
paints.

REACH requirements for component suppliers and equipment manufacturers
14 of 50
Substance Likely to be found in electrical and electronic equipment?
HBCDD
(hexabromocycl
ododecane)
Yes. HBCDD is used as an additive flame retardant in high impact
polystyrene (HIPS) which is found in electrical equipment including
housings and distribution boxes. Typical content range is 5% to 7%.
HBCDD is also used in expandable polystyrene (EPS) and extrudable
polystyrene (XPS).
SCCP (short-
chain
chlorinated
paraffins)
Possible. SCCP are currently used as a flame retardant in textiles and
rubber, in paint and in sealants and adhesives.
TBTO
(tributyltin
oxide)
Possible. TBTO is used at concentrations at about 1% in biocides used
in several manufacturing applications including polyurethane foam,
where it is added during the ‘blowing process’ and is subsequently
incorporated into the polymer matrix. An amendment to Annex XVII is
currently being prepared which will ban all uses of TBTO in articles.
Lead hydrogen
arsenate
No. Lead hydrogen arsenate was previously used as a pesticide.
Triethyl
arsenate
No. Although triethyl arsenate is used in the manufacturing process for
arsenide semiconductors, triethyl arsenate is not found in the
manufactured semiconductor components.
BBP
(benzylbutyl
phthalate)
Yes. BBP is one of the most expensive phthalates and so other
phthalates are generally used when possible. However, BBP is used as
a plasticiser in polymer products, mainly PVC. In flexible PVC the
typical phthalate content ranges from 30 to 45% w/w. BBP is also used
in certain sealants, adhesives, paints, inks and lacquers
8 Requirement for component suppliers to provide substance
declarations to manufacturers
Article 33 (1) requires any supplier of an article which contains more than 0.1% weight by
weight (w/w) of any substance on the Candidate List to provide the recipient of the article
with:
“sufficient information, available to the supplier, to allow safe use of the article
including, as a minimum, the name of that substance.”
“Recipient of an article” is defined in Article 3 (35) as
“Recipient of an article: means an industrial or professional user, or a distributor,
being supplied with an article but does not include consumers”

REACH requirements for component suppliers and equipment manufacturers
15 of 50
This obligation applies to all components, sub-assemblies and finished products which are
supplied to industrial or professional users, or distributors, in the EU. Take the example in
Figure 1 of a power supply unit which is manufactured by a contract manufacturer within
the EU. In this example, the legal obligation to inform the next recipient whether an article
contains more than 0.1% (w/w) of any substance on the Candidate List applies to:
• bare circuit boards manufactured by the board manufacturer;
• board-level components imported by the board-level component supplier;
• assembled circuit board manufactured by the PCB assembler;
• housing manufactured by the housing manufacturer;
• cables imported by the cable supplier;
• power supply unit assembled by the contract manufacturer
The ECHA press release published on 30 June
7
confirms that this legal obligation for all
EU component suppliers to inform manufacturers and distributors further down the supply
chain whether their components contain any of the Candidate List substances in
concentrations > 0.1% weight by weight (w/w) of the component starts from October 2008
when the first Candidate List was published.
REACH is fundamentally different to RoHS in this regard. Under RoHS, the legal
responsibility for ensuring compliance with the substance restrictions rests solely with the
OEM manufacturer or importer who puts the finished branded equipment
8
on the market in
the EU. There is no legal obligation on EU suppliers to provide information on the RoHS
compliance status of their components and sub-assemblies. As a result, OEM
manufacturers and importers incurred considerable costs to gather the necessary
compliance information from their suppliers. For example, some contract manufacturers
offer to gather this RoHS compliance information from suppliers as an additional business
service to their OEM clients.
In contrast, REACH places a legal obligation on all EU suppliers to provide substance
declaration information when they supply their articles (e.g. components and sub-
assemblies) to the next manufacturer in the supply chain. For example, this includes
contract manufacturers when they supply equipment to OEM clients, drawing on
information which component suppliers are required to disclose to the contract
manufacturer.
The substance declaration obligations also apply to the packaging materials, regardless of
whether it is manufactured in the EU or imported as part of imported goods. Packaging is
always treated as a separate ‘article’ under REACH and a separate substance declaration
is required for the packaging.
8.1 Supplier substance declarations
Starting from publication of the first Candidate List in October 2008, REACH places a legal
obligation on all EU component suppliers to inform manufacturers further down the supply
7
ECHA/PR/08/18, 30 June 2008
8
The RoHS substance restrictions apply to finished products which fall under Categories 1 to 7 and 10 of Annex I of the WEEE
Directive

REACH requirements for component suppliers and equipment manufacturers
16 of 50
chain whether their components contain any of the Candidate List substances in
concentrations > 0.1% weight by weight (w/w) of the component. Manufacturers should
insist that their suppliers comply with these legal obligations as this information enables
the manufacturers to make early strategic decisions on whether to design-out components
which contain these substances.
As discussed in section 6, many manufacturers will choose to phase out Candidate List
substances from their equipment for the following reasons:
• For uses not covered by a permanent exemption in Annex XIV, the continued
manufacturing of components in the EU which contain these substances will depend on
whether an authorisation is issued by the European Commission. There is no
guarantee that the Commission will issue an authorisation. Furthermore, each
authorisation will be for a limited time period after which the authorisation will be
reviewed and could be removed. Continued use of these components represents a
business risk to the manufacturer that the components may no longer be available in
the EU.
• To avoid this business risk, most companies will choose to phase out components
containing the Candidate List substances where possible. As a result, companies that
continue to use components containing these substances will find that they become
more expensive and have less availability.
• Starting from October 2008 REACH places a legal obligation on manufacturers and
importers to disclose to consumers on request whether their finished equipment
contains > 0.1% w/w of any Candidate List substance. This allows consumers to
choose whether to buy equipment which contain these substances of very high
concern. As consumer awareness of these substances increases, this will bring
increasing pressure for manufacturers to phase out the use of these substances.
Manufacturers also need this information from their suppliers to assist the manufacturers
to meet their legal obligations to provide information on Candidate List substances to
customers under REACH Article 33. If the customer is an industrial or professional user,
or a distributor, then Article 33 (1) requires the manufacturer to provide this information
when they supply their products. If the customer is a consumer (e.g. a private individual,
household user etc) then under Article 33 (2) the manufacturer is required to provide this
information free-of-charge within 45 days of receiving a request from any consumer (see
section 10).
8.2 Information on safe use
If a component does contain > 0.1% w/w of a Candidate List substance the component
supplier is also required to provide “sufficient information, available to the supplier, to allow
safe use of the article”. The ECHA Guidance
10
indicates that the component supplier
should consider what type of information and level of detail is appropriate to provide to the
next manufacturer down the supply chain. In the case where suppliers are providing
components for manufacturers to assemble into electrical and electronic equipment,
ENVIRON’s opinion is that it is sufficient to provide the substance name, CAS number and
risk phrase classification. This information is available to all suppliers, and enables the
manufacturer to take appropriate steps to ensure safe use of the component during the
10
Guidance on requirements for substances in articles, ECHA, May 2008

REACH requirements for component suppliers and equipment manufacturers
17 of 50
assembly process. An example of the information that should be provided by component
suppliers if their components contain > 0.1% w/w of DEHP is provided in Table 3.
Table 3: Information to be supplied with components which contain > 0.1% w/w DEHP
Substance name: di(ethylhexyl)phthalate (DEHP)
CAS. No: 117-81-7
Classification: Risk phrases R60-R61, toxic and toxic to reproduction
8.3 Ongoing supplier compliance requirements from October 2008
EU component suppliers have a legal obligation to provide substance declarations and
information on safe use immediately from the date that a substance is included in the
Candidate List. For the first list of substances, the press release issued by ECHA on 30
June confirms that these legal obligations started immediately when the Candidate List
was published in October 2008. More substances will be added to the Candidate List
every year by Member States, the European Commission and ECHA. For example, the
European Commission has already requested ECHA to prepare applications to add
another 5 substances to the Candidate List. In each case, the legal obligation to provide
substance declarations and information on safe use starts immediately the updated
Candidate List is published.
The obligation applies to any components that a supplier chooses to supply in the EU. For
example, spare parts, replacement items, upgrade kits etc. The only way that a
component supplier can avoid these obligations is to refuse to supply any components in
the EU.
The obligation applies to all components supplied in the EU after the date that the
substances were included on the Candidate List. Thus the date of supply of the article is
important. For example, these obligations apply to articles which were produced or
imported into the EU before the date that the substances were included on the Candidate
List and are supplied after the date of inclusion.
9 Substance disclosure requirements for imported assemblies
Section 2.2 and Section 2.3 of the ECHA Guidance
11
states that Article 33 (1) substance
disclosure requirements “apply to the article as produced or imported”. Austria, Belgium,
Denmark, France, Germany and Sweden have all refused to accept this application,
because it means that items which are assembled outside the EU and then imported into
the EU have different substance disclosure obligations under Article 33 (1) compared to
items which are assembled in the EU. This is illustrated in Figure 3 which is reproduced
from the ECHA Guidance.
For example, a power supply unit which is assembled outside the EU and then imported
into the EU is treated as one article. This means that the Article 33 (1) substance
disclosure requirements are based on whether Candidate List substances are present in
concentrations > 0.1% w/w of the whole power supply unit.
11
Guidance on Requirements for Substances in Articles, ECHA, May 2008

REACH requirements for component suppliers and equipment manufacturers
18 of 50
Figure 2: ECHA diagram which highlights how Article 33 (1) applies to equipment which is assembled
in the EU compared to equipment which is imported into the EU
In contrast, for a power supply unit which is assembled in the EU the Article 33 (1)
substance declaration requirements apply to each component supplier when they supply a
component to the next manufacturer in the supply chain. Using the example in Figure 1,
Article 7 (2) Notification and Article 33 Communication requirements will apply to the:
• bare circuit boards manufactured by the board manufacturer;
• board-level components imported by the board-level component supplier;
• assembled circuit board manufactured by the PCB assembler;
• housing manufactured by the housing manufacturer;
• cables imported by the cable supplier;
• power supply unit assembled by the contract manufacturer.
Whilst the majority of consumer products are assembled in South East Asia and imported
into the EU as whole products, it is common practice to also import spare parts,
replacement items and upgrade kits to support these products. Particularly for more
expensive business equipment, it is also common practice to provide component repairs to
this equipment locally in the EU, rather than shipping the whole product back for repair to
the country of manufacture. All of these components are classed as articles when they are
imported into the EU, or manufactured in the EU, and therefore must comply with Article
33 (1) when they are supplied to a recipient.

REACH requirements for component suppliers and equipment manufacturers
19 of 50
In practical terms, this means that EU manufacturers can leverage the legal obligations in
Article 33 (1) to require second, third, fourth etc tier upstream suppliers in the EU to
provide substance declarations for Candidate List substances. Manufacturers need this
level of detail to make early strategic decisions on whether to design-out components
which contain these substances.
For sub-assemblies and modules manufactured outside the EU and imported by suppliers,
manufacturers will need to put more pressure on their suppliers to provide this level of
detailed information. For example, where an EU manufacturer buys a power supply
module from an EU importer.
10 Requirement for manufacturers to provide substance declarations to
consumers on request
Starting from October 2008 REACH places a legal obligation on manufacturers and
importers to disclose to consumers on request whether their finished equipment contains >
0.1% w/w of any Candidate List substance. This allows consumers to choose whether to
buy equipment which contain these substances of very high concern. As consumer
awareness of these substances increases, this will bring increasing pressure for
manufacturers to phase out the use of these substances.
10.1 Substance declarations and information on safe use
Article 33 (2) requires any supplier of an article which contains more than 0.1% weight by
weight (w/w) of any substance on the Candidate List to provide the consumer on request
with:
“sufficient information, available to the supplier, to allow safe use of the article
including, as a minimum, the name of that substance.”
This information must be provided free-of-charge within 45 days of the supplier receiving a
request from any consumer.
The 0.1% w/w concentration applies to the article which is supplied to the consumer. For
example, if the supplier provides a bag of screws to a consumer for DIY applications then
the Article 33 (2) requirements apply to each individual screw. For an assembled item of
equipment like a TV set, the 0.1% w/w concentration applies to the weight of the TV. The
substance declaration obligations also apply to the packaging materials, regardless of
whether it is manufactured in the EU or imported as part of imported goods. Packaging is
always treated as a separate ‘article’ under REACH and a separate substance declaration
is required for the packaging.
The General Product Safety Directive 2001/95/EEC already includes legal obligations to
communicate information on product safety to consumers. This requirement is generally
met by providing safety information in the user manual or on the packaging. If a supplier’s
article does contain more than 0.1% w/w of any substance on the Candidate List then the
supplier should review the product safety information to ensure that it specifically takes
account of any necessary safety information relating to the Candidate List substance.
10.2 NGO action to promote consumer awareness of the Candidate List
NGO action to promote consumer awareness of REACH and the Candidate List has been
led by the European Environmental Bureau, Friends of the Earth and Greenpeace) who
banded together to form the Chemical Reaction project (www.chemicalreaction.org
).

REACH requirements for component suppliers and equipment manufacturers
20 of 50
Following the Chemical Reaction project, these NGOs started early to raise consumer
awareness of the right to enquire about the presence of Candidate List substances in
products and to urge companies to take early preparations, well in advance of publication
of the first Candidate List in October.
Greenpeace and Friends of the Earth publish an “Activists Guide to REACH” on their
websites which includes a template letter for consumers to use to request information
about Candidate List substances from suppliers, Figure 3.
Date
Dear Sir/Madam
In accordance with the new European regulation on Chemicals, REACH, I am
writing to ask you to inform me about the presence in the product XX or its
packaging of any chemical from the group of “substances of very high
concern” as specified by REACH.
Should any of these substances be present in the product XX or its
packaging, I wish to be informed about the name of this substance, and
receive sufficient information on how I can protect myself and the
environment from it.
I would be grateful to receive this information within 45 days as required
by REACH.
I would also be grateful if you would inform me about steps you are taking
to provide products intended for the same use but which do not contain such
potentially hazardous chemicals.
Yours faithfully,
cc: European Chemicals Agency – P.O.Box 400,00120 Helsinki, Finland
phone: +358-9-686180
email: [email protected], www.echa.europa.eu
Figure 3: Greenpeace and Friends of the Earth template letter for consumers to send to suppliers
11 Additional requirements for Candidate List substances in articles
11.1 Article 7 (2) notification of substances in articles
REACH also includes measures to enable ECHA to track the use of large quantities of
candidate list substances in articles. Article 7 (2) requires a company that manufactures or
imports articles into the EU to notify ECHA if all of the following conditions are met:
• The article contains a substance which is included in the Candidate List; and
• The Candidate List substance is present in the articles in quantities totaling over 1
tonne per manufacturer or importer per year; and
• The Candidate List substance is present in the articles in a concentration > 0.1% w/w
The requirement to provide notification to ECHA takes effect from 1 June 2011. The
deadline to provide notification to ECHA is six months after the substance has been
included in the Candidate List. The requirement to provide notification to ECHA does not
apply to articles which have already been manufactured in the EU, or imported into the EU,
before a substance is included on the Candidate List.

REACH requirements for component suppliers and equipment manufacturers
21 of 50
If, however, one or both of the following conditions are met then the manufacturer or
importer is not required to provide any notification:
• The manufacturer or importer can exclude exposure to humans or the environment
during normal or reasonably forseeable conditions of use including disposal (Article 7
(3)).
• The substance has already been registered for that use (Article 7 (6))
Note that the word ‘intended’ is not included in Article 7 (3). This means that notification to
the European Chemical Agency may be required if the substance can be foreseeably
released, even if unintentionally. One example of unintentional release is the wear of disk
brakes on a car which releases substances. This is not intentional (generally release due
to wear is not considered intentional under REACH) but if one of these substances is an
SVHC then it is possible that you may be required to notify the European Chemical Agency
if the above conditions are met. It is important to note that Article 7 (3) also includes
exposure to humans or the environment during disposal.
It is unlikely that all of the requirements listed above will be met on many occasions, in
particular that the substance has not already been registered for that use (Article 7 (6)). In
practice, therefore, the number of notifications by manufacturers and importers under
Article 7 (2) is likely to be low. However, there are some important substances that
manufacturers should pay particular attention to. For example, one of the substances on
the first Candidate List published in October 2008 is DEHP. Although over 400,000 tonnes
of DEHP is put on the EU market each year for use as a plasticiser in PVC, it is possible
that no one supplier puts more than 1,000 tonnes per year of DEHP on the market, in
which case this substance would not require registration for use as a PVC plasticiser until
May 2013. DEHP is used in flexible PVC applications in typical concentrations ranging
from 30 to 45% and it is well known that DEHP leaches out from PVC. In this case, a
company that is manufacturing or importing articles containing flexible PVC which come
into human contact would be required to submit a notification to ECHA in June 2011 if the
quantity of DEHP present in the articles was > 1 tonne per year.
The obligation to notify substances in articles also applies to the packaging materials,
which may be manufactured in the EU or imported as part of imported goods. Packaging
is always treated as a separate ‘article’ under REACH and the packaging must be
assessed separately from any object that it contains.
11.2 Article 66 notification of authorised substances in articles
Even if a component supplier or equipment manufacturer uses the substance in
accordance with one of the authorised uses, Article 66 still places obligations on the
component supplier or equipment manufacturer to notify ECHA. Article 66 requires all
component suppliers and equipment manufacturers who continue to use the substance in
accordance with an authorised use to notify ECHA within 3 months of the first supply of the
substance.
12 Substance restrictions enforced by REACH
The Marketing and Use Directive
12
was established in 1976 and places restrictions on the
manufacture and use of certain substances in the EU. Some of the substance restrictions
12
76/769/EC

REACH requirements for component suppliers and equipment manufacturers
22 of 50
are already covered by the RoHS Directive, for example the use of cadmium in paints,
plastics and coatings, and the use of lead carbonates and sulphates in paints. In addition,
the Marketing and Use Directive established a further 18 substance restrictions which are
potentially relevant to electrical and electronic equipment.
The European Commission decided to strengthen the implementation and enforcement of
these substance restrictions by including them in the REACH Regulation. Under REACH,
the European Commission can also place new restrictions on the manufacture of
substances, in addition to their marketing and use.
Article 67 of REACH replaces the current Marketing and Use Directive and specifies that
from June 2009
“A substance on its own, in a preparation, or in an article, shall not be
manufactured, placed on the market or used” unless it complies with the restrictions
in Annex XVII
“Placing on the market” is defined in Article 3 (12) as
“supplying or making available, whether in return for payment or free of charge, to a
third party. Import shall be deemed to be placing on the market.”
As per the current Marketing and Use Directive, there are 18 substance restrictions in
Annex XVII of the REACH Regulation which are potentially relevant to electrical and
electronic equipment, in addition to the substances which are already restricted under the
RoHS Directive.
12.1 Practical issues with the current Marketing and Use Directive
There are a number of practical issues with the way substances are currently restricted
under the current Marketing and Use Directive.
Implementation at Member State level through a variety of different regulatory approaches
Article 8 of the Marketing and Use Directive requires Member States to
“Bring into force the provisions necessary to comply with this Directive within 18
months of its notification and shall forthwith inform the Commission thereof”
In practice this has led to different Member States adopting a variety of different regulatory
approaches to implement the Directive. The Directive has been amended more than 30
times since 1976. In each case, the amendment has to be transposed into the different
regulatory approaches in each Member State. In the UK, for example, restrictions relating
to environmental protection were implemented by Regulations issued by DEFRA.
However, restrictions relating to worker protection were implemented by the Health and
Safety Executive and restrictions relating to consumer protection were implemented by the
Department for Business, Enterprise and Regulatory Reform (BERR). Up to 2007, DEFRA
had issued no less than 17 Regulations to implement the particular substance restrictions
that it was responsible for
13
!
13
In January 2007 DEFRA compiled all these restrictions into a single set of regulations, the Controls on Dangerous
Substances and Preparations Regulations 2006. However, these regulations will be replaced by REACH Article 67 from June
2009.

REACH requirements for component suppliers and equipment manufacturers
23 of 50
The situation is made worse because some Member States have adopted different
interpretations of key terms in the Directive in the implementation of their national
regulations.
In summary, under the current Marketing and Use Directive it is very hard for component
suppliers and equipment manufacturers to:
• identify the particular regulations they must comply with in each Member State;
• understand how key terms in the Marketing and Use Directive are interpreted in the
Member State’s regulations.
Different interpretations across Member States of who is responsible for compliance
Article 2 of the Marketing and Use Directive states:
“Member States shall take all necessary measures to ensure that the dangerous
substances and preparations listed in the Annex may only be placed on the market
or used subject to the conditions specified therein”.
However, “placing on the market” is not defined in the Directive and Member States are
free to interpret this concept provided the Treaty of Rome 1957 and other EC law is
respected. This has produced a confusing EC legal framework at national level
14
.
Some Member States have adopted the definition provided in the Commission Blue Guide
published in 1999 which defines “placing on the market” as
“the initial action of making a product available for the first time on the Community
Market with a view to distribution or use in the Community”.
The key difference between this definition and the definition in REACH is the word “first”.
In this definition, the notion of placing on the market is restricted to the ‘first making
available’. In Member States who adopted the Blue Guide definition, the competent
authorities cannot enforce the substance restrictions against subsequent acts of placing on
the Community market. Under REACH, however, enforcement can take place against all
subsequent acts of placing the article on the market.
12.2 Improved regulation of substance restrictions under REACH Article 67
Article 67 will improve the regulation and enforcement of these substances restrictions
from June 2009. Article 67 also clearly applies these substance restrictions to any
component supplier or equipment manufacturer that manufactures, imports or resells
components or equipment in the EU.
Single, uniform application across all EU Member States
REACH is an EU Regulation and applies uniformly across all Member States – it does not
require transposition into Member States regulatory approaches. This means that
component suppliers and equipment manufacturers will be required to comply directly with
the substance restrictions listed in Annex XVII.
14
The Interpretation of the term “placing on the market” in the context of the Marketing and Use Directive 76/769/EEC, Milieu
Environmental Law and Policy, February 2008

REACH requirements for component suppliers and equipment manufacturers
24 of 50
Clear identification of who is responsible for compliance
Article 67 states that from June 2009
“A substance on its own, in a preparation, or in an article, shall not be
manufactured, placed on the market or used” unless it complies with the
restrictions in Annex XVII
This makes component suppliers and equipment manufacturers responsible for
compliance with the substance restrictions when they manufacture or place an article on
the market. Article 3 (12) defines “placing on the market” as meaning
“supplying or making available, whether in return for payment or free of charge, to a
third party. Import shall be deemed to be placing on the market.”
In the example in Figure 1, the board manufacturer, PCB assembler, housing
manufacturer and contract manufacturer are all producers that place articles on the
market. Each of these component suppliers is responsible for compliance with the
substance restrictions in Annex XVII when they manufacture articles in the EU. In
this example, the component supplier and cable supplier are importers of articles which
are manufactured outside the EU. Each of these component suppliers is responsible
for compliance with the substance restrictions in Annex XVII when they import
articles into the EU.
Under the REACH definition of ‘placing on the market’, enforcement action can also
be taken against any organization that resells these articles in the EU.
Enforcement
Article 126 of REACH requires all Member States to define penalties for non-compliance
with any aspect of REACH, and report these to the European Commission by 1 December
2008. The penalties must be effective, proportionate and dissuasive and Member States
are responsible for taking all necessary steps to ensure that they are implemented.
Member States are required to appoint Competent Authorities with responsibility for
enforcing the penalties.
REACH also establishes an Enforcement Forum which is coordinated by ECHA. The
REACH Enforcement Forum is responsible for coordinating the different Member States’
Competent Authorities to ensure a consistent approach to enforcement across all Member
States.
Addition of new substance restrictions under Article 67
A request to include a new substance restriction under Article 67 can be initiated by any
Member State or ECHA (at the request of the Commission) by compiling a so-called
“Annex XV” dossier. The dossier must demonstrate that the risks from the substance are
not adequately controlled and request action to address this.
Member States have 12 months to prepare the dossier from the date that they notify
ECHA of their intention to prepare such as dossier, during which time another dossier for
the same substance can not be prepared by the Commission or another Member State.
ECHA is required to publish the Commission or Member State’s intention to launch a
restriction procedure for a substance, and to suggest a restriction within 12 months of
receiving a request from the Commission.

REACH requirements for component suppliers and equipment manufacturers
25 of 50
The final dossier and suggested restriction must be available for comment on the ECHA
website for 6 months. The Committee for Risk Assessment has to provide an opinion
within 9 months of the date of publication of the dossier, on whether the suggested
restriction will appropriately reduce the risk to human health and the environment. The
suggested restriction must also be considered by the Committee for Socio Economic
Analysis and subjected to further consultation, before the restriction is finally published.
12.3 REACH restricted substances that may be found in EEE
Article 67 refers to the substance restrictions listed in Annex XVII. In addition to
substances already restricted under the RoHS Directive (e.g. cadmium, lead), there are a
further 18 substance restrictions which are potentially relevant to electrical and electronic
equipment. ENVIRON has summarised these restrictions in Table 3 by grouping them
under common headings. This helps component suppliers and equipment manufacturers
to more quickly identify whether the substance restrictions are relevant to their
components or equipment.
Table 4: Article 67 (Annex XVII) substance restrictions which are potentially relevant to EEE
Substance Restriction
Asbestos No intentionally added content
Ozone depleting substances. (These are
liquids and gasses at room temperature)
Concentration must be less than 0.1% w/w in any
substance or preparation
Plasticisers
Phthalates (DEHP, DBP, BBP, DINP, DIDP,
DNOP)
Concentration must be < 0.1% w/w of plasticised
material when used in toys and childcare articles
Dielectric liquids previously used in transformers and capacitors
Polychlorinated biphenyls (PCBs) No content permitted
Polychlorinated terphenyls (PCTs) No content permitted
Monomethyl dibromodiphenyl methane (trade
name DBBT).
No content permitted
Monomethyl dichlorodiphenyl methane (trade
name Ugilec 121 or Ugilec 21).
No content permitted
Monomethyl tetrachlorodiphenyl methane
(trade name Ugilec 141).
No content permitted
Articles which may come into contact with skin
Azo colourants containing certain amines.
(These were previously used as dyes and
colourants)
Not permitted in textile and leather articles which
may come into direct and prolonged contact with
skin

REACH requirements for component suppliers and equipment manufacturers
26 of 50
Nickel and nickel alloys Must not be used in applications with direct and
prolonged skin contact and where the rate of
nickel release is > 0.5 micro gms per cm
2
per
week
Tri-(2,3-dibromo-propyl) phosphate Not permitted in textile articles which may come
into contact with skin
Tris-(1-aziridinyl) phosphinoxide Not permitted in textile articles which may come
into contact with skin
Biocides, pesticides, wood preservatives etc
Pentachlorophenol (PCP) and its salts and
compounds. (This was previously used as a
pesticide and wood preservative).
0.001%
Organostannic compounds. (These were
previously used as biocides and anti-oxidants
in paints)
No intentionally added content in substances or
preparations
Tar oils and creosotes No content permitted in wood
Other substances (all liquids at room temperature)
Benzene Concentration must be < 0.0005% w/w in toys and
< 0.1% w/w in other substances or preparations
Trichlorobenzene Concentration must be < 0.1% w/w in substances
or preparations
Nonylphenol and nonylphenol ethoxylates Concentration must be less than 0.1% w/w in
substances or preparations

REACH requirements for component suppliers and equipment manufacturers
27 of 50
13 BOMcheck web database to enable component suppliers to comply
with REACH requirements
As highlighted in the previous sections, REACH introduces new requirements on
component suppliers to provide substance declarations (Article 33) and comply with
substance restrictions (Article 67) when they supply their articles (e.g. components and
sub-assemblies) to the next manufacturer in the supply chain. REACH is fundamentally
different to RoHS in this regard.
Gathering and managing these substance declarations through the supply chain
represents a considerable challenge for manufacturers and suppliers. Suppliers need
guidance on the Candidate List substances and substance restrictions to help them make
their declarations to manufacturers. Manufacturers and suppliers need a system to help
them manage this information up the supply chain to the consumer, and keep up-to-date
with new SVHC substances as they are added to the Candidate List and new substance
restrictions added to Annex XVII. Manufacturers need to implement these systems early
so that they can plan for any required product design changes and minimise any disruption
to sales and maintenance of their equipment. The BOMcheck substances declarations
web database has been developed specifically to enable management of this information
through the supply chain.
ENVIRON and the COCIR medical device trade association believe the most cost-effective
and efficient approach is for suppliers and manufacturers to share one web-based system.
There are a number of existing web declarations systems (e.g. Synapsis EMARS, GEMS,
Technidata CfP etc). However, when ENVIRON and COCIR reviewed these systems we
found that none of them allow suppliers and manufacturers to share a single system.
Instead, these systems are focused on a RoHS-type approach where each manufacturer
purchases its own system to gather data separately from its own supply chain. We also
found that these existing web systems are very expensive. The most basic system costs
$60,000 in the first year and $30,000 per year thereafter. Some web systems cost up to
$300,000 in the first year!
COCIR therefore launched the BOMcheck substance declaration web database initiative
which will benefit not just the medical device industry but the whole electronics industry.
Developed by ENVIRON, the web database system reduces business costs by enabling
suppliers to upload their substances declarations to one location for access by all
participating manufacturers.
13.1 How is industry leading this initiative?
The BOMcheck initiative is led by COCIR (www.cocir.org
), the leading non-profit trade
association for the radiological, electromedical and healthcare IT industry in Europe.
COCIR membership comprises 14 of the largest manufacturers of electronic equipment in
the world, including GE, Siemens, Philips, Toshiba, Hitachi, Agfa and Intel. These
companies cover all sectors of the electronics industry. COCIR has also formed a
Steering Group to guide the development of BOMcheck, which is led by Siemens and
includes Philips, GE, Agfa and Texas Instruments.
The BOMcheck initiative was launched by COCIR at their offices in Brussels on 4 June
2008. The launch was attended by the European Commission and several other trade
associations.

REACH requirements for component suppliers and equipment manufacturers
28 of 50
Figure 4: BOMcheck substance declarations web database
“BOMcheck is the first system that allows electronics producers to reduce the industry
burden of ensuring REACH and RoHS compliance by compiling a centralised master
database of substance data from suppliers” said Freimut Schröder Siemens Healthcare
and COCIR Environmental Policy Focus Group chair.
COCIR Secretary General Nicole Denjoy added “This groundbreaking efficient BOMcheck
system reduces costs for our members and other industries as instead of each supplier
having to deal individually with each manufacturer, it will provide one central location
where the supplier can share its data with multiple manufacturers.”
The European Commission, Orgalime, European Lamp Companies Federation and Agoria
were among the delegates at the launch on 4 June in Brussels. The European
Commission congratulated COCIR for leading this initiative.

REACH requirements for component suppliers and equipment manufacturers
29 of 50
13.2 What are the benefits and costs to suppliers?
The primary purpose of BOMcheck is to enable suppliers to manage compliance with their
• provide Candidate List substance declarations from October 2008 (Article 33); and
• comply with substance restrictions from June 2009 (Article 67).
All EU suppliers are required to comply with these requirements when they supply articles
(i.e. components and sub-assemblies) to the next manufacturer in the supply chain. At the
same time, BOMcheck also enables suppliers to provide substance declarations for RoHS
and other restricted substances legislation.
Providing Candidate List substance declarations from October 2008
The Authorisation element of REACH is directly relevant to component suppliers and
equipment manufacturers as this will place limits on the use of an increasing number of
chemical substances. Under REACH, companies will need to apply for authorisation to
continue to use substances of very high concern (SVHC) which are included in the
Candidate List and then selected by ECHA.
Starting from October 2008 when the first Candidate List was published, REACH places a
legal obligation on all EU component suppliers to inform manufacturers further down the
supply chain whether their components contain any of the Candidate List substances in
concentrations > 0.1% weight by weight (w/w) of the component.
EU manufacturers insist that their EU suppliers comply with this legal requirement so that
the manufacturers can make early strategic decisions on whether to design-out
components which contain these substances.
Compliance with substance restrictions from June 2009
As explained in section 12.1 and 12.2, the new REACH definition of ‘placing on the market’
in Article 67 clarifies that from June 2009 enforcement action can be taken against any EU
organization which manufactures or supplies any article which does not comply with the
substance restrictions listed in REACH Annex XVII. This enforcement action can be taken
at any point in the supply chain. In particular, enforcement action can be taken against:
• an EU supplier which supplies components and sub-assemblies to a manufacturer that
do not comply with the substance restrictions
• an EU manufacturer which produces an article from these components and sub-
assemblies, which in turn does not comply with the substance restrictions.
To guard against this possible enforcement action, EU manufacturers will insist that their
EU suppliers provide declarations that the suppliers are meeting their legal obligations to
comply with the substance restrictions.
Cost-effective compliance with REACH requirements
BOMcheck provides a very cost-effective approach to enable suppliers to manage
compliance with their REACH requirements and provide substance declarations for RoHS.
An overview of the BOMcheck service for suppliers is provided in Figure 4.

REACH requirements for component suppliers and equipment manufacturers
30 of 50
The benefits to suppliers include:
• Guidance on Candidate List substances and substance restrictions to assist suppliers
to make their declarations to manufacturers. The on-line forms on BOMcheck highlight
where substances are commonly found in electrical and electronic equipment and
explain the restrictions and any exemptions that apply.
• For components that contain more than 0.1% w/w of a Candidate List substance,
BOMcheck automatically attaches the information on safe use which the supplier is
required to provide to the manufacturer.
• Electronic signature arrangements so that the suppliers electronic records on
BOMcheck are equivalent to paper records with handwritten signatures. BOMcheck
provides the necessary controls so that the suppliers electronic records comply with
Title 21 CFR Part 11 of the US Code of Federal Regulations under which the FDA
accept “electronic records and signatures as trustworthy, reliable and equivalent to
paper records with handwritten signatures executed in paper”.
• Keep up-to-date with REACH compliance requirements and RoHS restrictions.
ENVIRON provides regular updates to suppliers as more substances require
declaration (under REACH Article 33) or become restricted (under proposed
amendments to REACH Article 67 and RoHS). Suppliers can update their
declarations, submit additional declarations or withdraw part number declarations at
any time.
• BOMcheck quality management system processes and procedures are published on
www.BOMcheck.net
for review by manufacturers. This removes the need for suppliers
to develop and maintain their own quality management system for submitting
substance declarations to manufacturers. In many cases, responding to management
system questionnaires from manufacturers can be as time consuming as providing the
substance declarations.
• Time cost savings from using only one system to provide REACH and RoHS substance
declarations to multiple large electronics manufacturers.
In return for these benefits, suppliers pay a low-cost subscription of 300 Euros in the first
year and a reduced annual renewal fee in subsequent years. COCIR and ENVIRON
believe that this represents excellent value for money. The supplier pays the subscription
by credit card on www.BOMcheck.net
at the same time as it confirms the electronic
signature arrangements, Figure 6.
Most suppliers will join BOMcheck because they receive a letter from one of their
manufacturing customers informing them about REACH legal obligations in the EU, and
requiring them to comply with these requirements by joining BOMcheck, see Annex 3.
Once a supplier has joined BOMcheck they can use their membership to meet REACH
requirements for all of their other manufacturing customers as well. ENVIRON provides a
letter that suppliers can send to all their manufacturing customers (see Annex 4) to:
• Confirm that the supplier is a member of BOMcheck and that the supplier has followed
the BOMcheck guidance and procedures to provide its REACH and RoHS substance
declarations on www.BOMcheck.net
• Highlight that BOMcheck is free for manufacturers provided they send a letter to their
own suppliers to require them to comply with their REACH requirements by joining
BOMcheck.

REACH requirements for component suppliers and equipment manufacturers
31 of 50
• Emphasize that the supplier’s electronic records on BOMcheck include electronic
signatures so that they are equivalent to paper records with handwritten signatures
Many suppliers are also manufacturers and have their own supply chains. These suppliers
can set up a manufacturer account with BOMcheck to manage receipt of REACH and
RoHS substance declarations from their own suppliers. As explained in section 13.3,
BOMcheck is free for manufacturers provided they send a letter to their suppliers to require
them to comply with their REACH requirements by joining BOMcheck. For example, the
contract manufacturer described in Figure 1 can use BOMcheck to receive REACH and
RoHS substance declarations from the EU suppliers of cables, housings, circuit boards etc
which it assembles into the power supply unit.
The REACH compliance benefits and low cost / free use of BOMcheck by suppliers and
manufacturers enables BOMcheck to cascade through the supply chain, Figure 5.
Figure 5: How BOMcheck cascades REACH compliance through the supply chain
Manufacturing
customer A
Manufacturing
customer B
Supplier 1
Supplier (a)
Supplier (b)
Supplier 2
Sends letters requiring
suppliers to join BOMcheck
Sends BOMcheck
membership letter
Sends BOMcheck
membership letter
Sends letters requiring
suppliers to join BOMcheck
Sends letters requiring
suppliers to join BOMcheck

REACH requirements for component suppliers and equipment manufacturers
32 of 50
Figure 6: BOMcheck service for suppliers

REACH requirements for component suppliers and equipment manufacturers
33 of 49
13.3 What are the benefits and costs to manufacturers?
The main benefit that BOMcheck provides to manufacturers is a high quality, robust and
cost-effective process for managing the REACH substance declarations which suppliers
are required to provide to:
• Comply with the Article 33 (1) obligations to disclose the presence of Candidate List
substance in articles from October 2008; and
• Demonstrate compliance with substance restrictions from June 2009 (Article 67).
This enables the manufacturers to comply with their REACH obligations under Article 33
and Article 67 when they supply assembled products onto their customers, and also to
take early strategic decisions on phase-out of Candidate List substances. At the same
time, BOMcheck also enables suppliers to provide substance declarations for RoHS and
other restricted substances legislation.
Providing Candidate List substance declarations from October 2008
In addition to obligations on suppliers, REACH Article 33 also places obligations on
manufacturers to inform their customers whether the assembled product contains more
than 0.1% w/w of any Candidate List substance.
If the customer is an industrial or professional user, or a distributor, then Article 33 (1)
requires the manufacturer to provide this information when they supply their products.
If the customer is a consumer (e.g. a private individual, household user etc) then under
Article 33 (2) the manufacturer is required to provide this information free-of-charge within
45 days of receiving a request from any consumer.
To enable them to comply with this REACH requirement, all EU manufacturers should
insist that their EU suppliers comply with their legal obligations under Article 33 (1) to
inform them whether the components and sub-assemblies they supply contain Candidate
List substances.
BOMcheck gathers the weights of the suppliers’ parts and the actual percentage
composition of Candidate List substances in the parts. The manufacturer can use
BOMcheck to calculate whether a Bill of Materials (a structured list of part numbers) for a
particular product exceeds the 0.1% threshold for any Candidate List substance. No
manufacturer product data is stored on BOMcheck. The manufacturer’s list of part
numbers is automatically deleted after the calculations have been completed on
BOMcheck.
Taking early strategic decisions on Candidate List substances from October 2008
Starting from October 2008 when the first Candidate List was published, REACH Article 33
(1) places a legal obligation on all EU component suppliers to inform manufacturers further
down the supply chain whether their components contain any of the Candidate List
substances in concentrations > 0.1% weight by weight (w/w) of the component.
All EU manufacturers insist that their EU suppliers comply with their legal obligations under
Article 33 (1) so that the manufacturers can make early strategic decisions on whether to
design-out components which contain Candidate List substances. As discussed in section
6, many manufacturers will choose to phase out Candidate List substances from their
equipment for the following reasons:

REACH requirements for component suppliers and equipment manufacturers
34 of 49
• For uses not covered by a permanent exemption in Annex XIV, the continued
manufacturing of components in the EU which contain priority substances drawn from
the Candidate List will depend on whether an authorisation is issued by the European
Commission. There is no guarantee that the Commission will issue an authorisation.
Furthermore, each authorisation will be for a limited time period after which the
authorisation will be reviewed and could be removed. Continued use of these
components represents a business risk to the manufacturer that the components may
no longer be available in the EU.
• To avoid this business risk, most companies will choose to phase out components
containing the Candidate List substances where possible. As a result, companies that
continue to use components containing these substances will find that they become
more expensive and have less availability.
• Starting from October 2008 REACH places a legal obligation on manufacturers and
importers to disclose to consumers on request whether their finished equipment
contains > 0.1% w/w of any Candidate List substance. This allows consumers to
choose whether to buy equipment which contain these substances of very high
concern. As consumer awareness of these substances increases, this will bring
increasing pressure for manufacturers to phase out the use of these substances.
EU manufacturers can leverage the legal obligations in Article 33 (1) to require second,
third, fourth etc tier upstream suppliers in the EU to provide substance declarations for
Candidate List substances. For sub-assemblies and modules manufactured outside the
EU and imported by suppliers, manufacturers will need to put more pressure on their
suppliers to provide this level of detailed information. For example, where an EU
manufacturer buys a power supply module from an EU importer.
Manufacturers can choose to store a confidential ‘watch list’ of part numbers on
BOMcheck and receive automatic notification if:
• A supplier changes the declaration status of any part number on the watch list. For
example, in response to a new substance being added to the Candidate List or
becoming restricted under Article 67
• A RoHS exemption which the part number relies on is removed by the European
Commission (for example, in the case of the recent removal of the exemption for deca-
BDE)
This ‘watch list’ is stored in a secure area on BOMcheck which can not be accessed by
any user.
Managing compliance with substance restrictions from June 2009
As explained in section 12.1 and 12.2, the new REACH definition of ‘placing on the market’
in Article 67 clarifies that from June 2009 enforcement action can be taken against any EU
organization which manufactures or supplies any article which does not comply with the
substance restrictions listed in REACH Annex XVII. This enforcement action can be taken
at any point in the supply chain. In particular, enforcement action can be taken against:
• an EU supplier which supplies components and sub-assemblies to a manufacturer that
do not comply with the substance restrictions
• an EU manufacturer which produces an article from these components and sub-
assemblies, which in turn does on comply with the substance restrictions.

REACH requirements for component suppliers and equipment manufacturers
35 of 49
To guard against this possible enforcement action, EU manufacturers should insist that
their EU suppliers provide declarations that the suppliers are meeting their legal
obligations to comply with the substance restrictions. This legal obligation also applies to
suppliers who import sub-assemblies and modules manufactured outside the EU.
Free membership of BOMcheck for manufacturers
Membership of BOMcheck is free for manufacturers provided they agree to send a letter to
their suppliers to require them to comply with their REACH requirements by joining
BOMcheck. ENVIRON provides a standard letter which manufacturers can adapt and use,
see Annex 3. The manufacturer must agree to send the letter within 3 months to all of its
suppliers (including suppliers on the manufacturer’s Approved Supplier Lists or
equivalent).
Each supplier pays a low-cost subscription of 300 Euros per year to join BOMcheck. The
supplier pays their subscription by credit card on BOMcheck.net at the same time as it
confirms the electronic signature arrangements. ENVIRON will provide free membership
of BOMcheck for small suppliers with a turnover of less than 3,000,000 Euros.
In effect, by agreeing to send out the letter the manufacturer is agreeing to ‘market’ the
BOMcheck service to its suppliers. ENVIRON and COCIR believe that BOMcheck
represents an extremely cost-effective approach to enable suppliers to comply with
REACH substance declaration requirements. We believe that manufacturers sending out
the letter will be sufficient for suppliers to appreciate these benefits and decide to join
BOMcheck. In addition, we recognize that it is in the manufacturer’s interests to ensure
that all of its suppliers join BOMcheck. Accordingly, ENVIRON does not audit whether the
manufacturer has sent out the letter to all suppliers or whether all suppliers have joined
BONcheck. Instead, we rely on the BOMcheck benefits to be self-evident to suppliers and
manufacturers alike, and for market forces to prevail.
The BOMcheck service for manufacturers is summarized in Figure 7. To request a
manufacturer’s agreement please contact Dr Aidan Turnbull Head of WEEE, RoHS &
EcoDesign.
Email: [email protected]vironcorp.com
Telephone: +44 (0)1225 748420
High quality and robust process for managing REACH and RoHS substance declarations
Manufacturers gain peace of mind from knowing that their suppliers are following a high
quality and robust process for providing substance declarations for REACH and RoHS. An
overview of the BOMcheck service for suppliers is provided in Figure 6. The benefits
include:
• Guidance on Candidate List substances and substance restrictions to assist suppliers
to make their declarations to manufacturers. The on-line forms on BOMcheck highlight
where substances are commonly found in electrical and electronic equipment and
explain the restrictions and any exemptions that apply.
• For components that contain more than 0.1% w/w of a Candidate List substance,
BOMcheck automatically attaches the information on safe use which the supplier is
required to provide to the manufacturer.

REACH requirements for component suppliers and equipment manufacturers
36 of 49
• Electronic signature arrangements so that the suppliers electronic records on
BOMcheck are equivalent to paper records with handwritten signatures. BOMcheck
provides the necessary controls so that the suppliers electronic records comply with
Title 21 CFR Part 11 of the US Code of Federal Regulations under which the FDA
accept “electronic records and signatures as trustworthy, reliable and equivalent to
paper records with handwritten signatures executed in paper”.
• Keep up-to-date with REACH compliance requirements and RoHS restrictions.
ENVIRON provides regular updates to suppliers as more substances require
declaration (under REACH Article 33) or become restricted (under proposed
amendments to REACH Article 67 and RoHS). Suppliers can update their
declarations, submit additional declarations or withdraw part number declarations at
any time.
• BOMcheck quality management system processes and procedures are published on
www.BOMcheck.net
for review by manufacturers. This removes the need for suppliers
to develop and maintain their own quality management system for submitting
substance declarations to manufacturers.

REACH requirements for component suppliers and equipment manufacturers
37 of 49
Figure 7: BOMcheck service for manufacturers

REACH requirements for component suppliers and equipment manufacturers
38 of 49
14 Managing RoHS compliance
In addition to providing substance declarations to comply with their REACH obligations
under Article 33 and Article 67, BOMcheck also enables suppliers to provide substance
declarations for RoHS and other restricted substances legislation.
14.1 How does BOMcheck manage changes in RoHS exemptions?
If a supplier claims that the use of certain substances in their parts is exempted under the
RoHS Directive, then BOMcheck requires the supplier to select which particular exemption
the supplier is claiming. If a particular exemption is removed from the RoHS Directive,
BOMcheck will require all supplier members that relied on the exemption to update their
substance declarations to indicate whether their parts still comply.
The recent removal of the Deca BDE exemption is a good example. Many manufacturers
had not gathered sufficient information from their suppliers and so had to re-contact their
suppliers to confirm whether they had relied on this exemption.
14.2 How does BOMcheck manage compliance with RoHS restrictions in China,
Korea, Japan?
Other RoHS legislation around the world focuses on the same list of RoHS substances,
but has different requirements. BOMcheck analyses the substance declarations provided
by suppliers to identify any restrictions on using these parts in other parts of the world.
China RoHS
The Management Methods of Controlling Pollution by Electronic Information Products,
known as China RoHS, is being implemented in two phases. It addresses the same
substances as the EC RoHS Directive, but includes a much larger scope of products.
Similar to the EC RoHS Directive, additional substances may also be covered under China
RoHS in the future.
The first phase took effect from 1 March 2007 and comprises a series of marking
requirements. In addition to a label on the product, if the product contains RoHS materials
then the user manual must also include a Hazardous Substance Table (in Chinese) which
lists where these substances are found in the product. Another marking requirement,
described in Article 14, requires the manufacturer to label the packaging and to use non-
toxic, harmless, readily degradable and recyclable materials.
The second phase is when the actual substance restrictions will take effect. In 2008 the
Chinese government will publish a ‘catalog’ which will list the dates by which particular
products must comply with the substance restrictions. This may include exemptions from
materials restrictions for certain types of equipment and in certain applications. The
catalog will also identify some products where pre-market testing and certification will be
required before the product can be sold in China.
Korea RoHS
The Act for Resource Recycling of Electrical/Electric Products and Automobiles was
published on 2 April 2007 and came into force on 1 January 2008. The Act applies the
same EC RoHS materials restrictions and maximum concentration values to 10 categories
of electrical and electronic equipment which are listed in Article 6, Enforcement Ordinance
of the Act.

REACH requirements for component suppliers and equipment manufacturers
39 of 49
Japan RoHS
Under an amendment to the Law for the Promotion of the Effective Utilisation of
Resources, Japan introduced a mandatory labelling standard for certain types of
household electrical equipment and IT equipment from 1 July 2006. If any single
homogenous material in these types of equipment contains > 0.01% by weight of cadmium
or > 0.1% by weight of lead, mercury, hexavalent chromium, PBB or PBDE, the J-MOSS
labeling standard requires that the equipment is marked with an orange “R” mark. If the
equipment does not contain these materials it should be marked with a green “G” mark.
14.3 What are the new substances that may be added to RoHS?
ENVIRON represented the Medical Device Industry at the European Commission
stakeholder workshop on 6 May 2008 to discuss possible new substances to be added to
the RoHS Directive. The Commission’s proposal for a new RoHS Directive was issued for
inter-service consultation on 29 September. There is no immediate extension to the list of
restricted substances under RoHS. Instead, the proposal for a new RoHS Directive
includes a new Annex C which lists the following 5 substances to be assessed for further
possible restriction under the RoHS Directive:
• TBBP-A (tetrabromobisphenol-A) as an additive flame retardant. Use of TBBP-A as a
reactive flame retardant in epoxy and polycarbonate resins would be exempted.
TBBP-A is widely used as a reactive flame retardant in printed circuit boards. The
main use of TBBP-A as an additive flame retardant is in ABS (acrylonitrile-butadiene-
styrene) resins.
• HBCDD (hexabromocyclododecane). HBCDD is used as a flame retardant in HIPS
(high impact polystyrenes)
• Three types of phthalates: DEHP (bis[2-ethylhexyl] phthalate), BPP (butyl benzyl
phthalate) and DBP (dibutylphthalate). These are mainly used as plasticizers in
polymer products, particularly PVC. A number of exemptions would be required for
particular materials applications, for example in medical devices.
Apart from TBBP-A, the other four substances are already included in the first Candidate
List published in October 2008.
Once the Commission assessment has confirmed that these new substances will be added
to the RoHS Directive, we will notify all supplier members that they must now provide
declarations for these substances for their part numbers at the single homogenous
material level. The on-line forms on BOMcheck will help suppliers to complete their
substances declarations by:
• explaining the restrictions that apply to each new RoHS substance and any exemptions
that apply
• highlighting where the new RoHS substances are commonly found in electrical and
electronic equipment
• providing information on alternative substances, where available.

REACH requirements for component suppliers and equipment manufacturers
40 of 49
15 About ENVIRON
ENVIRON is a leading international environmental and life sciences consultancy with 1,300
staff in 73 offices across 16 countries. Business turnover in 2007 was US $200,000,000.
Further details about ENVIRON can be found at www.environcorp.com
.
The BOMcheck service is delivered by ENVIRON’s EcoDesign Practice which has
extensive experience in delivering REACH, RoHS, WEEE and EcoDesign services for the
electronic equipment industry. Highlights include:
• ENVIRON has extensive experience in all aspects of the REACH Directive. In
particular, we have carried out REACH gap analysis and developed REACH
compliance action plans for several large electronic equipment manufacturers. For
more information about this service contact Dr Aidan Turnbull on
[email protected]oncorp.com
.
• ENVIRON are the EcoDesign consultant for the leading European trade association
COCIR (www.cocir.org
) and represent the Medical Device Industry in negotiations with
the European Commission on the RoHS Directive. ENVIRON has written several joint
industry statements on proposals for new RoHS substances and possible inclusion of
medical devices in RoHS.
• ENVIRON provided detailed consulting advice to the Irish WEEE Agency
(www.WEEERegister.ie
) to assist with the practical implementation of the Irish WEEE
Regulations for B2B products.
ENVIRON also provides dedicated WEEE compliance services for producers of Business-
to-Business (B2B) electrical and electronic equipment.
• Waste Electrical and Electronic Equipment (WEEE) compliance requirements are
different in each EU Member State. But ENVIRON's web-based compliance
management systems (www.b2bweee.com
) enable manufacturers and importers of
business products to cut through the confusion and have a single system for WEEE
collection, recycling and reporting across Europe.
• The UK WEEE Regulations require importers and manufactures to join an approved
WEEE Producer Compliance Scheme for producer registration and compliance data
reporting to the Environment Agency. ENVIRON’s Scheme (www.b2bweee-
scheme.com) was the first fully approved Scheme in the UK.
ENVIRON’s EcoDesign practice is led by Dr Aidan Turnbull. Aidan holds a Doctorate in
Physics and Electronics, sponsored by British Telecom through a CASE award. He taught
undergraduate electronics at Durham University and Glasgow University. He is also a
registered Environmental Auditor with the Institute of Environmental Management and
Assessment (IEMA).
Aidan is an internationally recognised expert on EcoDesign and has won consulting
awards in the UK
15
and US
16
. Aidan's specialist expertise in EcoDesign in the electronics
sector results from a wide range of projects over the past 10 years ranging from
15
Surface Engineering Association Outstanding Achievement Award for 2004, AEA Technology Project Manager of the Year
1996
16
Environment Business Journal Project Merit Award 2004

REACH requirements for component suppliers and equipment manufacturers
41 of 49
international projects with Blue Chip companies, Government departments and agencies,
and leading industry trade associations. Aidan was one of only eight eco-design
consultants appointed to a UK Government panel that advises on both compliance and
broader eco-design approaches. Aidan also managed and wrote the UK Government
Guidelines on actions that companies should take to prepare for compliance with WEEE
and RoHS. Separate guidelines were produced for CEOs, Marketing Directors and
Technical Directors, and Designers. The guidelines were published by the Government
Agency Envirowise in 2003.
16 Further information
Suppliers can join BOMcheck by completing the on-line application forms on
www.bomcheck.net
.
Manufacturers can request a free web demonstration or receive a manufacturer agreement
for action by contacting:
Europe and US
Dr Aidan Turnbull, Head of WEEE, RoHS & EcoDesign.
Email: [email protected]vironcorp.com
Telephone: +44 (0) 1249 700104
Japan and Asia Pacific
Dr Kanji Tamamushi
Email: ktamamushi@environcorp.com
Telephone: +81 90 1212 1476

REACH requirements for component suppliers and equipment manufacturers
42 of 49
ANNEX 1: Key REACH Articles for equipment manufacturers / importers
Article 7: Registration and notification of substances in articles
1. Any producer or importer of articles shall submit a registration to the Agency for any substance
contained in those articles, if both the following conditions are met:
(a) the substance is present in those articles in quantities totalling over 1 tonne per
producer or importer per year;
(b) the substance is intended to be released under normal or reasonably foreseeable
conditions of use.
A submission for registration shall be accompanied by the fee required in accordance Title IX.
2. Any producer or importer of articles shall notify the Agency, in accordance with paragraph 4 of
this Article, if a substance meets the criteria in Article 57 and is identified in accordance with
Article 59(1), if both the following conditions are met:
(a) the substance is present in those articles in quantities totalling over 1 tonne per
producer or importer per year;
(b) the substance is present in those articles above a concentration of 0,1 % weight by
weight (w/w).
3. Paragraph 2 shall not apply where the producer or importer can exclude exposure to humans or
the environment during normal or reasonably foreseeable conditions of use including disposal.
In such cases, the producer or importer shall supply appropriate instructions to the recipient of
the article.
4. The information to be notified shall include the following:
(a) the identity and contact details of the producer or importer as specified in section 1 of
Annex VI, with the exception of their own use sites;
(b) the registration number(s) referred to in Article 20(1), if available;
(c) the identity of the substance as specified in sections 2.1 to 2.3.4 of Annex VI;
(d) the classification of the substance(s) as specified in sections 4.1 and 4.2 of Annex VI;
(e) a brief description of the use(s) of the substance(s) in the article as specified in section
3.5 of Annex VI and of the uses of the article(s);
(f) the tonnage range of the substance(s), such as 1-10 tonnes, 10-100 tonnes and so on.
5. The Agency may take decisions requiring producers or importers of articles to submit a
registration, in accordance with this Title, for any substance in those articles, if all the following
conditions are met:
(a) the substance is present in those articles in quantities totalling over 1 tonne per
producer or importer per year;
(b) the Agency has grounds for suspecting that:
i) the substance is released from the articles, and
ii) the release of the substance from the articles presents a risk to human health
or the environment;
(c) the substance is not subject to paragraph 1.
A submission for registration shall be accompanied by the fee required in accordance Title IX.
6. Paragraphs 1 to 5 shall not apply to substances that have already been registered for that use.
7. From 1 June 2011 paragraphs 2, 3 and 4 of this Article shall apply 6 months after a substance
is identified in accordance with Article 59(1).
8. Any measures for the implementation of paragraphs 1 to 7 shall be adopted in accordance with
the procedure referred to in Article 133(3).

REACH requirements for component suppliers and equipment manufacturers
43 of 49
Article 33: Duty to communicate information on substances in articles
1. Any supplier of an article containing a substance meeting the criteria in Article 57 and identified
in accordance with Article 59(1) in a concentration above 0.1 % weight by weight (w/w) shall
provide the recipient of the article with sufficient information, available to the supplier, to allow
safe use of the article including, as a minimum, the name of that substance.
2. On request by a consumer any supplier of an article containing a substance meeting the criteria
in Article 57 and identified in accordance with Article 59(1) in a concentration above 0,1 %
weight by weight (w/w) shall provide the consumer with sufficient information, available to the
supplier, to allow safe use of the article including, as a minimum, the name of that substance.
The relevant information shall be provided, free of charge, within 45 days of receipt of the
request.
Article 67: Restrictions on the manufacturing, placing on the market and use of
certain dangerous substances, preparations and articles
1. A substance on its own, in a preparation or in an article, for which Annex XVII contains a
restriction shall not be manufactured, placed on the market or used unless it complies with the
conditions of that restriction. This shall not apply to the manufacture, placing on the market or
use of a substance in scientific research and development. Annex XVII shall specify if the
restriction shall not apply to product and process orientated research and development, as well
as the maximum quantity exempted.
2. Paragraph 1 shall not apply to the use of substances in cosmetic products, as defined by
Directive 76/768/EEC, with regard to restrictions addressing the risks to human health within
the scope of that Directive.
3. Until 1 June 2013, a Member State may maintain any existing and more stringent restrictions in
relation to Annex XVII on the manufacture, placing on the market or use of a substance,
provided that those restrictions have been notified according to the Treaty. The Commission
shall compile and publish an inventory of these restrictions by 1 June 2009.

REACH requirements for component suppliers and equipment manufacturers
44 of 49
ANNEX 2: Safety Data Sheet or similar safety information requirements
for supply of substances and preparations purchased in the EU
In some cases, a component supplier or equipment manufacturer may purchase substances or
preparations in the EU for supply or use with their components or equipment. In this case,
component suppliers and equipment manufacturers should check that they are using the
substances or preparations in a way which has already been identified by an upstream supplier in
the EU. If the component supplier or equipment manufacturer is supplying or using the substance
in a way which has not been identified by an upstream supplier, they can choose to:
• Inform the supplier under Article 37 (2) and require them to address this use in their registration
dossier for the substance under Article 37 (3). Provided the supplier is informed at least 12
months before the registration deadline, the supplier is required to support the use, unless the
use represents unacceptable risk to human health or the environment;
• Keep this use of the substance confidential in which case the component supplier or equipment
manufacturer must prepare a chemical safety report under Article 37 (4).
Component suppliers or equipment manufacturer who purchase substances or preparations in the
EU for supply or use with their components or equipment will also need to ensure the following
requirements are addressed:
• Article 31: Requirements for Safety Data Sheets
• Article 32: Duty to communicate information down the supply chain for substances on their own
or in preparations for which a safety data sheet is not required
• Article 34: Duty to communicate information on substances and preparations up the supply
chain
• Access to information for workers and documentation retention obligations in Articles 35 and 36
The component supplier or equipment manufacturer should require their supplier to provide all
necessary information to meet Article 31 and Article 32 requirements. The component supplier or
equipment manufacturer should communicate this information to their customers, to meet Article 34
requirements. The main requirements in these Articles are summarised below.
REACH Article 31 (1) requires the supplier of a substance or preparation to provide the recipient of
the substance or preparation with a safety data sheet where:
• a substance or preparation is classified as dangerous according to the Dangerous Substances
Directive or the Dangerous Preparations Directive
17
; or
• a substance is PBT (persistent, bioaccumulative and toxic) or vPvB (very persistent and very
bioaccumulative) in accordance with the criteria set out in Annex XIII; or
• a substance is included in the Candidate List as per Article 59 (1)
Article 31 (2) requires the supplier to provide the recipient at his request with a safety data sheet
where a preparation is not classified as dangerous according to the Dangerous Preparations
Directive, but contains:
• in an individual concentration of >
1 % by weight for non-gaseous preparations (and > 0.2% by
volume for gaseous preparations) at least one substance posing human health or
environmental hazards; or
17
Directive 1999/45/EC

REACH requirements for component suppliers and equipment manufacturers
45 of 49
• in an individual concentration of > 0.1% by weight for non-gaseous preparations at least one
substance that is PBT (persistent, bioaccumulative and toxic) or vPvB (very persistent and very
bioaccumulative) in accordance with the criteria set out in Annex XIII or is included in the
Candidate List as per Article 59 (1)
• a substance for which there are Community workplace exposure limits.
Article 32 requires the supplier of a substance or preparation to provide the recipient with certain
other substance information, even when a safety data sheet is not required.
Article 34 requires any manufacturer or importer or downstream user of a substance or preparation
to communicate information to the next recipient of the substance or preparation.
Are there any requirements to supply safety data sheets for articles?
As discussed above, Article 31 places certain requirements for a supplier who provides substances
or preparations to a recipient to provide that recipient with a safety data sheet. Article 32 requires
the supplier of a substance or preparation to provide the recipient with certain other substance
information, even when a safety data sheet is not required. In both these cases, the requirements
only relate to a supplier who provides substances or preparations to a recipient. This is several
stages down the supply chain from the manufacturer of the finished item of electrical or electronic
equipment. For example, take an FR4 circuit board which is made from an epoxy resin. The
supplier of the epoxy resin (a preparation) is required to provide a safety data sheet (or other
substance information required under Article 32) to the company who manufactures the circuit
board (an article). As the circuit board is an article (and not a substance or a preparation) there is
no requirement for the circuit board manufacturer to provide a safety data sheet (or other substance
information required under Article 32) when the board is then sold to a printed circuit board
assembler. The circuit board assembler then sells the populated circuit board to the electrical or
electronic equipment manufacturer (or other tier in the supply chain), again without a safety data
sheet (or other substance information required under Article 32).

REACH requirements for component suppliers and equipment manufacturers
46 of 49
ANNEX 3: BOMcheck letter for manufacturers to send to suppliers
[Supplier Name]
[Supplier address]
[Date]
Dear [name]
RE: Requirement to comply with EU REACH obligations by joining the BOMcheck substances
declarations web database
As soon as the first Candidate List is published in October 2008, Article 33 (1) of the REACH Regulation places
a legal obligation on all EU suppliers to inform [Manufacturer name] whether the components or assemblies
supplied to us contain any of the REACH Candidate List substances in concentrations > 0.1% w/w. For all
components or assemblies which exceed this concentration, the supplier has a legal obligation to provide
information to [Manufacturer name] on safe use.
From June 2009, Article 67 of REACH places a legal obligation on all EU suppliers to comply with the
substance restrictions listed in Annex XVII when they supply components or sub-assemblies to [Manufacturer
name].
In view of these obligations, [Manufacturer name] requires all of our suppliers (including Suppliers on the
[Manufacturer name] Approved Supplier Lists) to join the BOMcheck substances declarations web database
(www.BOMcheck.net
). BOMcheck is an industry-wide initiative which is driven by the European trade
association COCIR (www.cocir.org
) and has been developed by ENVIRON. Membership of the COCIR trade
association includes Siemens, Philips, GE, Agfa, Hitachi, Toshiba, IBM, Intel and Canon.
The web database covers REACH, RoHS and other restricted substances legislation. The benefits to suppliers
include:
• Guidance on Candidate List substances and substance restrictions to assist suppliers to make their
declarations to manufacturers. The on-line forms on BOMcheck highlight where substances are
commonly found in electrical and electronic equipment and explain the restrictions and any exemptions
that apply.
• For components that contain more than 0.1% w/w of a Candidate List substance, BOMcheck
automatically attaches the information on safe use which the supplier is required to provide to the
manufacturer.
• Electronic signature arrangements so that the suppliers electronic records on BOMcheck are equivalent to
paper records with handwritten signatures. BOMcheck provides the necessary controls so that the
suppliers electronic records comply with Title 21 CFR Part 11 of the US Code of Federal Regulations
under which the FDA accept “electronic records and signatures as trustworthy, reliable and equivalent
to paper records with handwritten signatures executed in paper”.
• Keep up-to-date with REACH compliance requirements and RoHS restrictions. ENVIRON provides
regular updates to suppliers as more substances require declaration (under REACH Article 33) or become
restricted (under proposed amendments to REACH Article 67 and RoHS). Suppliers can update their
declarations, submit additional declarations or withdraw part number declarations at any time.
• BOMcheck quality management system processes and procedures are published on www.BOMcheck.net
for review by manufacturers. This removes the need for suppliers to develop and maintain their own
quality management system for submitting substance declarations to manufacturers.
• Time cost savings from using only one system to provide REACH and RoHS substance declarations to
multiple large electronics manufacturers.
In return for these benefits, suppliers pay a low-cost subscription of 300 Euros year in the first year and a
reduced annual renewal fee in subsequent years. We believe that this represents excellent value for money.

REACH requirements for component suppliers and equipment manufacturers
47 of 49
Once a supplier has joined BOMcheck they can use their membership to demonstrate compliance with
REACH obligations to other manufacturing customers. ENVIRON provides a letter that suppliers can send
to all their manufacturing customers to:
• Confirm that the supplier is a member of BOMcheck and that the supplier has followed the BOMcheck
guidance and procedures to provide its REACH and RoHS substance declarations on
www.BOMcheck.net
• Highlight that BOMcheck is free for manufacturers provided they send a letter to their own suppliers to
require them to comply with REACH obligations by joining BOMcheck;
• Emphasize that the supplier’s electronic records on BOMcheck include electronic signatures so that they
are equivalent to paper records with handwritten signatures.
Many suppliers are also manufacturers and have their own supply chains. These suppliers can set up a
manufacturer account with BOMcheck to manage receipt of REACH and RoHS substance declarations from
their own suppliers.
Please give us notice by returning to us a signed copy of this letter whether you will join BOMcheck. When
you join BOMcheck you will conclude a separate contract with ENVIRON by following the instructions at
www.BOMcheck.net
. Further details are available at www.bomcheck.net/supplier.
We look forward to your cooperation in this important matter.
Yours sincerely
Our company will / will not join BOMcheck [delete as
applicable]
Notified by [supplier name]
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
[signature]
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
[name]
. . . . . . . . . . . . . . . . . . . . . . . . . . . .
[title]

REACH requirements for component suppliers and equipment manufacturers
48 of 49
ANNEX 4: BOMcheck letter for suppliers to send to manufacturers
[Manufacturer Name]
[Manufacturer address]
[Date]
Dear [name]
RE: [Supplier Name] compliance with EU REACH requirements through BOMcheck substances
declarations web database
This is to inform [Manufacturer Name] how you can access the substance declaration information which
[Supplier Name] provides to meet requirements under the EU REACH Regulation.
As soon as the first Candidate List is published in October 2008, Article 33 (1) of the REACH Regulation places
a legal obligation on all EU suppliers to inform their manufacturing customers whether the components or
assemblies they supply contain any of the REACH Candidate List substances in concentrations > 0.1% w/w.
For all components or assemblies which exceed this concentration, the supplier has a legal obligation to provide
information on safe use.
From June 2009, Article 67 of REACH places a legal obligation on all EU suppliers to comply with the
substance restrictions listed in Annex XVII when they supply components or sub-assemblies to manufacturing
customers.
To meet these requirements, [Supplier Name] has joined the BOMcheck substances declarations web database.
BOMcheck is an industry-wide initiative which is driven by the European trade association COCIR
(www.cocir.org
) and has been developed by ENVIRON. Membership of the COCIR trade association includes
Siemens, Philips, GE, Agfa, Hitachi, Toshiba, IBM, Intel and Canon.
[Supplier Name] has followed the procedures and guidance on BOMcheck to provide substance declarations for
REACH, RoHS and other restricted substances legislation on www.BOMcheck.net
. [Supplier Name] complies
with all of the BOMcheck Member Rules and ENVIRON has certified our electronic signature arrangements
with the US FDA. Accordingly, our electronic records on BOMcheck comply with Title 21 CFR Part 11 of the
US Code of Federal Regulations under which the FDA accept “electronic records and signatures as
trustworthy, reliable and equivalent to paper records with handwritten signatures executed in paper”.
We pay a low-cost subscription of 300 Euros per year to use the BOMcheck service. We believe this represents
excellent value for money.
BOMcheck is free for manufacturers provided they send a letter to their own suppliers to require them to
comply with REACH obligations by joining BOMcheck. To request a manufacturer’s agreement to join
BOMcheck please contact Dr Aidan Turnbull, Head of WEEE, RoHS and EcoDesign, ENVIRON, e-mail:
, tel: +44 1225 748420.
Manufacturers benefit from knowing that their suppliers are following a high quality and robust process for
providing substances declarations for REACH and RoHS. BOMcheck quality management system processes
and procedures are published on www.BOMcheck.net
. The benefits to manufacturers also include:
• BOMcheck gathers the weights of the suppliers’ parts and the actual percentage composition of
Candidate List substances in the parts. The manufacturer can use BOMcheck to calculate whether a Bill
of Materials (a structured list of part numbers) for a particular product exceeds the 0.1% threshold for
any Candidate List substance. No manufacturer product data is stored on BOMcheck. The
manufacturer’s list of part numbers is automatically deleted after the calculations have been completed
on BOMcheck.

REACH requirements for component suppliers and equipment manufacturers
49 of 49
• Manufacturers can choose to store a confidential ‘watch list’ of part numbers on BOMcheck and receive
automatic notification if:
– A supplier changes the declaration status of any part number on the watch list. For example, in
response to a new substance being added to the Candidate List or becoming restricted under
Article 67
– A RoHS exemption which the part number relies on is removed by the European Commission (for
example, in the case of the recent removal of the exemption for deca-BDE)
• Keep up-to-date with REACH compliance requirements and RoHS restrictions. ENVIRON provides
regular updates to suppliers as more substances require declaration (under REACH Article 33) or become
restricted (under proposed amendments to REACH Article 67 and RoHS). Suppliers can update their
declarations on BOMcheck, submit additional declarations or withdraw part number declarations at any
time.
• Time cost savings from using only one system to access to REACH and RoHS substance declarations
from multiple suppliers.
Yours sincerely
